Structural Heterogeneity of the GABAergic Tripartite Synapse
Abstract
:1. Introduction
2. Results
2.1. Expansion Microscopy of GABAergic Synapses and Perisynaptic Astroglia
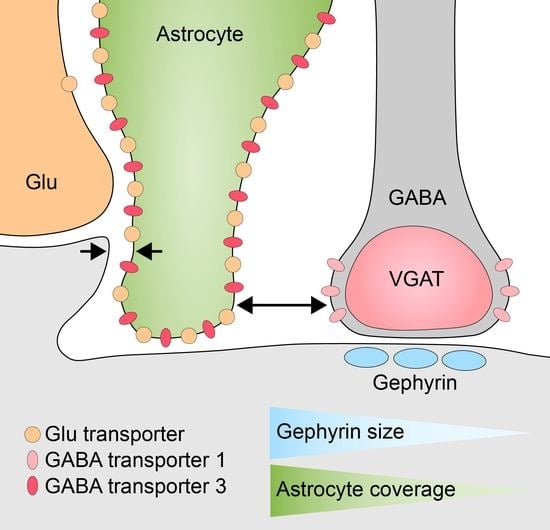
2.2. Astrocytic Volume Distribution at GABAergic and Glutamatergic Synapses
2.3. GABAergic Synapse Properties Correlate with Local Astrocytic Process Distribution
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Expansion Microscopy
4.3. Image Analysis
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mederos, S.; Perea, G. GABAergic-Astrocyte Signaling: A Refinement of Inhibitory Brain Networks. Glia 2019, 67, 1842–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishibashi, M.; Egawa, K.; Fukuda, A. Diverse Actions of Astrocytes in GABAergic Signaling. Int. J. Mol. Sci. 2019, 20, 2964. [Google Scholar] [CrossRef] [Green Version]
- Ribak, C.E.; Tong, W.M.Y.; Brecha, N.C. GABA Plasma Membrane Transporters, GAT-1 and GAT-3, Display Different Distributions in the Rat Hippocampus. J. Comp. Neurol. 1996, 367, 595–606. [Google Scholar] [CrossRef]
- Melone, M.; Ciappelloni, S.; Conti, F. Plasma Membrane Transporters GAT-1 and GAT-3 Contribute to Heterogeneity of GABAergic Synapses in Neocortex. Front. Neuroanat. 2014, 8, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, I.; Volynski, K.; Brenner, T.; Ushkaryov, Y.; Walker, M.; Semyanov, A. Different Transporter Systems Regulate Extracellular GABA from Vesicular and Non-Vesicular Sources. Front. Cell. Neurosci. 2013, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Doengi, M.; Hirnet, D.; Coulon, P.; Pape, H.-C.; Deitmer, J.W.; Lohr, C. GABA Uptake-Dependent Ca2+ Signaling in Developing Olfactory Bulb Astrocytes. Proc. Natl. Acad. Sci. USA 2009, 106, 17570–17575. [Google Scholar] [CrossRef] [Green Version]
- Serrano, A.; Haddjeri, N.; Lacaille, J.-C.; Robitaille, R. GABAergic Network Activation of Glial Cells Underlies Hippocampal Heterosynaptic Depression. J. Neurosci. 2006, 26, 5370–5382. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Jiang, L.; Goldman, S.A.; Nedergaard, M. Astrocyte-Mediated Potentiation of Inhibitory Synaptic Transmission. Nat. Neurosci. 1998, 1, 683–692. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, B.-E.; Berglund, K.; Oh, S.-J.; Park, H.; Shin, H.-S.; Augustine, G.J.; Lee, C.J. Channel-Mediated Tonic GABA Release from Glia. Science 2010, 330, 790–796. [Google Scholar] [CrossRef]
- Lee, M.; McGeer, E.G.; McGeer, P.L. Mechanisms of GABA Release from Human Astrocytes. Glia 2011, 59, 1600–1611. [Google Scholar] [CrossRef]
- Le Meur, K.; Mendizabal-Zubiaga, J.; Grandes, P.; Audinat, E. GABA Release by Hippocampal Astrocytes. Front. Comput. Neurosci. 2012, 6, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aten, S.; Kiyoshi, C.M.; Arzola, E.P.; Patterson, J.A.; Taylor, A.T.; Du, Y.; Guiher, A.M.; Philip, M.; Camacho, E.G.; Mediratta, D.; et al. Ultrastructural View of Astrocyte Arborization, Astrocyte-Astrocyte and Astrocyte-Synapse Contacts, Intracellular Vesicle-like Structures, and Mitochondrial Network. Prog. Neurobiol. 2022, 213, 102264. [Google Scholar] [CrossRef] [PubMed]
- Herde, M.K.; Bohmbach, K.; Domingos, C.; Vana, N.; Komorowska-Müller, J.A.; Passlick, S.; Schwarz, I.; Jackson, C.J.; Dietrich, D.; Schwarz, M.K.; et al. Local Efficacy of Glutamate Uptake Decreases with Synapse Size. Cell Rep. 2020, 32, 108182. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, N.; Popov, V.; Henneberger, C.; Kraev, I.; Rusakov, D.A.; Stewart, M.G. Glia Selectively Approach Synapses on Thin Dendritic Spines. Phil. Trans. R. Soc. B 2014, 369, 20140047. [Google Scholar] [CrossRef] [Green Version]
- Patrushev, I.; Gavrilov, N.; Turlapov, V.; Semyanov, A. Subcellular Location of Astrocytic Calcium Stores Favors Extrasynaptic Neuron-Astrocyte Communication. Cell Calcium 2013, 54, 343–349. [Google Scholar] [CrossRef]
- Ventura, R.; Harris, K.M. Three-Dimensional Relationships between Hippocampal Synapses and Astrocytes. J. Neurosci. 1999, 19, 6897–6906. [Google Scholar] [CrossRef]
- Witcher, M.R.; Park, Y.D.; Lee, M.R.; Sharma, S.; Harris, K.M.; Kirov, S.A. Three-Dimensional Relationships between Perisynaptic Astroglia and Human Hippocampal Synapses. Glia 2010, 58, 572–587. [Google Scholar] [CrossRef] [Green Version]
- Bernardinelli, Y.; Randall, J.; Janett, E.; Nikonenko, I.; König, S.; Jones, E.V.; Flores, C.E.; Murai, K.K.; Bochet, C.G.; Holtmaat, A.; et al. Activity-Dependent Structural Plasticity of Perisynaptic Astrocytic Domains Promotes Excitatory Synapse Stability. Curr. Biol. 2014, 24, 1679–1688. [Google Scholar] [CrossRef] [Green Version]
- Haber, M.; Zhou, L.; Murai, K.K. Cooperative Astrocyte and Dendritic Spine Dynamics at Hippocampal Excitatory Synapses. J. Neurosci. 2006, 26, 8881–8891. [Google Scholar] [CrossRef] [Green Version]
- Henneberger, C.; Bard, L.; Panatier, A.; Reynolds, J.P.; Kopach, O.; Medvedev, N.I.; Minge, D.; Herde, M.K.; Anders, S.; Kraev, I.; et al. LTP Induction Boosts Glutamate Spillover by Driving Withdrawal of Perisynaptic Astroglia. Neuron 2020, 108, 919–936.e11. [Google Scholar] [CrossRef]
- Lushnikova, I.; Skibo, G.; Muller, D.; Nikonenko, I. Synaptic Potentiation Induces Increased Glial Coverage of Excitatory Synapses in CA1 Hippocampus. Hippocampus 2009, 19, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Perez-Alvarez, A.; Navarrete, M.; Covelo, A.; Martin, E.D.; Araque, A. Structural and Functional Plasticity of Astrocyte Processes and Dendritic Spine Interactions. J. Neurosci. 2014, 34, 12738–12744. [Google Scholar] [CrossRef] [PubMed]
- Witcher, M.R.; Kirov, S.A.; Harris, K.M. Plasticity of Perisynaptic Astroglia during Synaptogenesis in the Mature Rat Hippocampus. Glia 2007, 55, 13–23. [Google Scholar] [CrossRef]
- Kikuchi, T.; Gonzalez-Soriano, J.; Kastanauskaite, A.; Benavides-Piccione, R.; Merchan-Perez, A.; DeFelipe, J.; Blazquez-Llorca, L. Volume Electron Microscopy Study of the Relationship Between Synapses and Astrocytes in the Developing Rat Somatosensory Cortex. Cereb. Cortex 2020, 30, 3800–3819. [Google Scholar] [CrossRef] [PubMed]
- Megías, M.; Emri, Z.; Freund, T.F.; Gulyás, A.I. Total Number and Distribution of Inhibitory and Excitatory Synapses on Hippocampal CA1 Pyramidal Cells. Neuroscience 2001, 102, 527–540. [Google Scholar] [CrossRef]
- Rusakov, D.A.; Bard, L.; Stewart, M.G.; Henneberger, C. Diversity of Astroglial Functions Alludes to Subcellular Specialisation. Trends Neurosci. 2014, 37, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tillberg, P.W.; Boyden, E.S. Expansion Microscopy. Science 2015, 347, 543–548. [Google Scholar] [CrossRef] [Green Version]
- Chozinski, T.J.; Halpern, A.R.; Okawa, H.; Kim, H.-J.; Tremel, G.J.; Wong, R.O.L.; Vaughan, J.C. Expansion Microscopy with Conventional Antibodies and Fluorescent Proteins. Nat. Methods 2016, 13, 485–488. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, T.; Li, T.; Herde, M.K.; Becker, A.; Vatter, H.; Schwarz, M.K.; Henneberger, C.; Steinhäuser, C.; Bedner, P. Subcellular Reorganization and Altered Phosphorylation of the Astrocytic Gap Junction Protein Connexin43 in Human and Experimental Temporal Lobe Epilepsy. Glia 2017, 65, 1809–1820. [Google Scholar] [CrossRef]
- Minge, D.; Domingos, C.; Unichenko, P.; Behringer, C.; Pauletti, A.; Anders, S.; Herde, M.K.; Delekate, A.; Gulakova, P.; Schoch, S.; et al. Heterogeneity and Development of Fine Astrocyte Morphology Captured by Diffraction-Limited Microscopy. Front. Cell Neurosci. 2021, 15, 669280. [Google Scholar] [CrossRef]
- Chaudhry, F.A.; Reimer, R.J.; Bellocchio, E.E.; Danbolt, N.C.; Osen, K.K.; Edwards, R.H.; Storm-Mathisen, J. The Vesicular GABA Transporter, VGAT, Localizes to Synaptic Vesicles in Sets of Glycinergic as Well as GABAergic Neurons. J. Neurosci. 1998, 18, 9733–9750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.-L.; Gong, N. Glycine and Glycine Receptor Signaling in Hippocampal Neurons: Diversity, Function and Regulation. Prog. Neurobiol. 2010, 91, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Tyagarajan, S.K.; Fritschy, J.-M. Gephyrin: A Master Regulator of Neuronal Function? Nat. Rev. Neurosci. 2014, 15, 141–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolte, C.; Matyash, M.; Pivneva, T.; Schipke, C.G.; Ohlemeyer, C.; Hanisch, U.K.; Kirchhoff, F.; Kettenmann, H. GFAP Promoter-Controlled EGFP-Expressing Transgenic Mice: A Tool to Visualize Astrocytes and Astrogliosis in Living Brain Tissue. Glia 2001, 33, 72–86. [Google Scholar] [CrossRef]
- Tyagarajan, S.K.; Ghosh, H.; Yévenes, G.E.; Nikonenko, I.; Ebeling, C.; Schwerdel, C.; Sidler, C.; Zeilhofer, H.U.; Gerrits, B.; Muller, D.; et al. Regulation of GABAergic Synapse Formation and Plasticity by GSK3β-Dependent Phosphorylation of Gephyrin. Proc. Natl. Acad. Sci. USA 2011, 108, 379–384. [Google Scholar] [CrossRef] [Green Version]
- Santuy, A.; Tomás-Roca, L.; Rodríguez, J.-R.; González-Soriano, J.; Zhu, F.; Qiu, Z.; Grant, S.G.N.; DeFelipe, J.; Merchan-Perez, A. Estimation of the Number of Synapses in the Hippocampus and Brain-Wide by Volume Electron Microscopy and Genetic Labeling. Sci. Rep. 2020, 10, 14014. [Google Scholar] [CrossRef]
- Specht, C.G.; Izeddin, I.; Rodriguez, P.C.; El Beheiry, M.; Rostaing, P.; Darzacq, X.; Dahan, M.; Triller, A. Quantitative Nanoscopy of Inhibitory Synapses: Counting Gephyrin Molecules and Receptor Binding Sites. Neuron 2013, 79, 308–321. [Google Scholar] [CrossRef] [Green Version]
- Pennacchietti, F.; Vascon, S.; Nieus, T.; Rosillo, C.; Das, S.; Tyagarajan, S.K.; Diaspro, A.; Bue, A.D.; Petrini, E.M.; Barberis, A.; et al. Nanoscale Molecular Reorganization of the Inhibitory Postsynaptic Density Is a Determinant of GABAergic Synaptic Potentiation. J. Neurosci. 2017, 37, 1747–1756. [Google Scholar] [CrossRef] [Green Version]
- Crosby, K.C.; Gookin, S.E.; Garcia, J.D.; Hahm, K.M.; Dell’Acqua, M.L.; Smith, K.R. Nanoscale Subsynaptic Domains Underlie the Organization of the Inhibitory Synapse. Cell Rep. 2019, 26, 3284–3297.e3. [Google Scholar] [CrossRef] [Green Version]
- Rusakov, D.A.; Davies, H.A.; Harrison, E.; Diana, G.; Richter-Levin, G.; Bliss, T.V.P.; Stewart, M.G. Ultrastructural Synaptic Correlates of Spatial Learning in Rat Hippocampus. Neuroscience 1997, 80, 69–77. [Google Scholar] [CrossRef]
- Harris, K.M.; Stevens, J.K. Dendritic Spines of CA 1 Pyramidal Cells in the Rat Hippocampus: Serial Electron Microscopy with Reference to Their Biophysical Characteristics. J. Neurosci. 1989, 9, 2982–2997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schikorski, T.; Stevens, C.F. Quantitative Ultrastructural Analysis of Hippocampal Excitatory Synapses. J. Neurosci. 1997, 17, 5858–5867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubota, Y.; Kondo, S.; Nomura, M.; Hatada, S.; Yamaguchi, N.; Mohamed, A.A.; Karube, F.; Lübke, J.; Kawaguchi, Y. Functional Effects of Distinct Innervation Styles of Pyramidal Cells by Fast Spiking Cortical Interneurons. eLife 2015, 4, e07919. [Google Scholar] [CrossRef] [PubMed]
- Korogod, N.; Petersen, C.C.; Knott, G.W. Ultrastructural Analysis of Adult Mouse Neocortex Comparing Aldehyde Perfusion with Cryo Fixation. eLife 2015, 4, e05793. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Danbolt, N. GABA and Glutamate Transporters in Brain. Front. Endocrinol. 2013, 4, 165. [Google Scholar] [CrossRef] [Green Version]
- Minelli, A.; Brecha, N.C.; Karschin, C.; DeBiasi, S.; Conti, F. GAT-1, a High-Affinity GABA Plasma Membrane Transporter, Is Localized to Neurons and Astroglia in the Cerebral Cortex. J. Neurosci. 1995, 15, 7734–7746. [Google Scholar] [CrossRef]
- Rose, C.R.; Felix, L.; Zeug, A.; Dietrich, D.; Reiner, A.; Henneberger, C. Astroglial Glutamate Signaling and Uptake in the Hippocampus. Front. Mol. Neurosci. 2017, 10, 451. [Google Scholar] [CrossRef] [Green Version]
- Nusser, Z.; Hájos, N.; Somogyi, P.; Mody, I. Increased Number of Synaptic GABAA Receptors Underlies Potentiation at Hippocampal Inhibitory Synapses. Nature 1998, 395, 172–177. [Google Scholar] [CrossRef]
- Flores, C.E.; Méndez, P. Shaping Inhibition: Activity Dependent Structural Plasticity of GABAergic Synapses. Front. Cell. Neurosci. 2014, 8, 327. [Google Scholar] [CrossRef]
- Klausberger, T.; Somogyi, P. Neuronal Diversity and Temporal Dynamics: The Unity of Hippocampal Circuit Operations. Science 2008, 321, 53–57. [Google Scholar] [CrossRef]
- Matthews, E.A.; Sun, W.; McMahon, S.M.; Doengi, M.; Halka, L.; Anders, S.; Müller, J.A.; Steinlein, P.; Vana, N.S.; van Dyk, G.; et al. Optical Analysis of Glutamate Spread in the Neuropil. Cereb. Cortex 2022, 32, bhab440. [Google Scholar] [CrossRef] [PubMed]
- Asano, S.M.; Gao, R.; Wassie, A.T.; Tillberg, P.W.; Chen, F.; Boyden, E.S. Expansion Microscopy: Protocols for Imaging Proteins and RNA in Cells and Tissues. Curr. Protoc. Cell Biol. 2018, 80, e56. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunskine, C.; Passlick, S.; Henneberger, C. Structural Heterogeneity of the GABAergic Tripartite Synapse. Cells 2022, 11, 3150. https://doi.org/10.3390/cells11193150
Brunskine C, Passlick S, Henneberger C. Structural Heterogeneity of the GABAergic Tripartite Synapse. Cells. 2022; 11(19):3150. https://doi.org/10.3390/cells11193150
Chicago/Turabian StyleBrunskine, Cindy, Stefan Passlick, and Christian Henneberger. 2022. "Structural Heterogeneity of the GABAergic Tripartite Synapse" Cells 11, no. 19: 3150. https://doi.org/10.3390/cells11193150