Species-Specific Gamete Interaction during Sea Urchin Fertilization: Roles of the Egg Jelly and Vitelline Layer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gametes Collection, Egg Jelly, and Vitelline Layer Removal and Fertilization Procedure
2.2. Scanning and Transmission Electron Microscopy (SEM & TEM)
2.3. Microinjection, Ca2+ Imaging, and Confocal Microscopy
2.4. Visualization of Sperm inside the Eggs upon Insemination
2.5. Statistical Analysis
3. Results
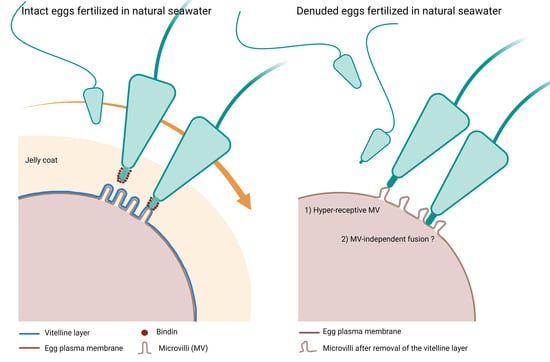
3.1. The Removal of the Egg Jelly and Vitelline Layer of P. lividus Eggs Has a Striking Effect on the Egg Surface Morphology
3.2. Alteration of the Cortical Reaction upon Insemination of P. lividus Eggs Deprived of the Jelly and Vitelline Layers
3.3. P. lividus Eggs Fertilized in Seawater at pH 5.5 or in NSW after DTT Treatment at pH 9 Show an Altered Sperm-Egg Binding and Entry into the Eggs
3.4. The Removal of the Vitelline Layer Strongly Alters the Ca2+ Response at Fertilization
3.5. The Plasma Membrane of Denuded Eggs of P. lividus Does Not Fuse with Arbacia lixula Sperm
3.6. Alteration of the Cortical Actin Polymerization and Translocation following Incubation and Insemination of P. lividus Eggs in Acidic Seawater
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dan, J.C. Studies on the acrosome. III. Effect of calcium deficiency. Biol. Bull. 1954, 107, 335–349. [Google Scholar] [CrossRef]
- Tilney, L.G.; Hatano, S.; Ishikawa, H.; Mooseker, M.S. The polymerization of actin: Its role in the generation of the acrosomal process of certain echinoderm sperm. J. Cell Biol. 1973, 59, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Chen, S.H.; Zhou, H.; Vacquier, V.D. Tandem mass spectrometry identifies proteins phosphorylated by cyclic AMP-dependent protein kinase when sea urchin sperm undergo the acrosome reaction. Dev. Biol. 2005, 285, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Darszon, A.; Guerrero, A.; Galindo, B.E.; Nishigaki, T.; Wood, C.D. Sperm-activating peptides in the regulation of ion fluxes, signal transduction and motility. Int. J. Dev. Biol. 2008, 52, 595–606. [Google Scholar] [CrossRef]
- Chun, J.T.; Vasilev, F.; Limatola, N.; Santella, L. Fertilization in Starfish and Sea Urchin: Roles of Actin. Cell Differ. 2018, 65, 33–47. [Google Scholar] [CrossRef]
- Collins, F.; Epel, D. The role of calcium ions in the acrosome reaction of sea urchin sperm: Regulation of exocytosis. Exp. Cell Res. 1977, 106, 211–222. [Google Scholar] [CrossRef]
- García-Soto, J.; Darszon, A. High pH-induced acrosome reaction and Ca2+ uptake in sea urchin sperm suspended in Na+-free seawater. Dev. Biol. 1985, 110, 338–345. [Google Scholar] [CrossRef]
- Dan, J.C. Studies on the acrosome. I. Reaction to egg water and other stimuli. Biol. Bull. 1952, 103, 54–66. [Google Scholar] [CrossRef]
- Lillie, F.R. The production of sperm-isoagglutinins by ova. Science 1912, 36, 527–530. [Google Scholar] [CrossRef]
- Hagström, B. Studies on the fertilization of jelly-free sea urchin eggs. Exp. Cell Res. 1956, 10, 24–28. [Google Scholar] [CrossRef]
- Aketa, K.; Ohta, T. When do sperm of the sea urchin, Pseudocentrotus depressus, undergo the acrosome reaction at fertilization? Dev. Biol. 1977, 61, 366–372. [Google Scholar] [CrossRef]
- Vacquier, V.D.; Brandriff, B.; Glabe, C.G. The effect of soluble egg jelly on the fertilizability of acid-dejeleld sea urchin eggs. Develop. Growth Differ. 1979, 21, 47–60. [Google Scholar] [CrossRef]
- Hirohashi, N.; Vilela-Silva, A.-C.E.S.; Mourão, P.A.S.; Vacquier, V.D. Structural requirements for specie specific induction of the sperm acrosome reaction by sea urchin egg sulfated fucan. Biochem. Biophys. Res. Commun. 2002, 298, 403–407. [Google Scholar] [CrossRef]
- Vacquier, V.D.; Moy, G.W. Isolation of bindin: The protein responsible for adhesion of sperm to sea urchin eggs. Proc. Natl. Acad. Sci. USA 1977, 74, 2456–2460. [Google Scholar] [CrossRef] [PubMed]
- Glabe, C.G. Interaction of the sperm adhesive protein, bindin, with phospholipid vesicles. II Bindin induces the fusion of mixed-phase vsicles that contain phosphatidylcholine and phosphatidilseryne in vitro. J. Cell Biol. 1985, 100, 800–806. [Google Scholar] [CrossRef]
- Vacquier, V.D. The quest for the sea urchin egg receptor for sperm. Biochem. Biophys. Res. Commun. 2012, 425, 583–587. [Google Scholar] [CrossRef]
- Aketa, K.; Onitake, K.; Tsuzuki, H. Tryptic disruption of sperm-binding site of sea urchin egg surface. Exp. Cell Res. 1972, 71, 27–32. [Google Scholar] [CrossRef]
- Lallier, R. Effects of concanavalin on the development of sea urchin egg. Exp. Cell Res. 1972, 72, 157–163. [Google Scholar] [CrossRef]
- Veron, M.; Shapiro, B.M. Binding of concanavalin A to the surface of sea urchin eggs and its alteration upon fertilization. J. Biol. Chem. 1977, 252, 1286–1292. [Google Scholar] [CrossRef]
- Longo, F.J. Effects of concanavalin A on the fertilization of sea urchin eggs. Dev. Biol. 1981, 82, 197–202. [Google Scholar] [CrossRef]
- Wessel, G.M.; Wada, Y.; Yajima, M.; Kiyomoto, M. Bindin is essential for fertilization in the sea urchin. Proc. Natl. Acad. Sci. USA 2021, 118, e2109636118. [Google Scholar] [CrossRef] [PubMed]
- Hagström, B.; Hagström, B. A method of determining the fertilization rate in sea urchins. Exp. Cell Res. 1954, 6, 479–484. [Google Scholar] [CrossRef]
- Presley, R.; Baker, P.F. Kinetics of fertilization in the sea urchin: A comparison of methods. J. Exp. Biol. 1970, 52, 455–468. [Google Scholar] [CrossRef]
- Vacquier, V.D.; Payne, J.E. Methods for quantitating sea urchin sperm-egg binding. Exp. Cell Res. 1973, 82, 227–235. [Google Scholar] [CrossRef]
- Limatola, N.; Chun, J.T.; Cherraben, S.; Schmitt, J.L.; Lehn, J.M.; Santella, L. Effects of dithiothreitol on fertilization and early development in sea urchin. Cells. 2021, 10, 3573. [Google Scholar] [CrossRef]
- Epel, D.; Weaver, A.M.; Mazia, D. Methods for removal of the vitelline membrane of sea urchin eggs. Exp. Cell Res. 1970, 61, 64–68. [Google Scholar] [CrossRef]
- Chun, J.T.; Limatola, N.; Vasilev, F.; Santella, L. Early events of fertilization in sea urchin eggs are sensitive to actin-binding organic molecules. Biochem. Biophys. Res. Commun. 2014, 450, 1166–1174. [Google Scholar] [CrossRef]
- Vasilev, F.; Limatola, N.; Chun, J.T.; Santella, L. Contributions of suboolemmal acidic vesicles and microvilli to the intracellular Ca2+ increase in the sea urchin eggs at fertilization. Int. J. Biol. Sci. 2019, 15, 757–775. [Google Scholar] [CrossRef]
- Yonemura, S.; Mabuchi, I. Wave of cortical actin polymerization in the sea urchin egg. Cell Motil. Cytoskelet. 1987, 7, 46–53. [Google Scholar] [CrossRef]
- Terasaki, M. Actin filament translocations in sea urchin eggs. Cell Motil. Cytoskelet. 1996, 34, 48–56. [Google Scholar] [CrossRef]
- Limatola, N.; Vasilev, F.; Chun, J.T.; Santella, L. Sodium-mediated fast electrical depolarization does not prevent polyspermic fertilization in Paracentrotus lividus eggs. Zygote 2019, 27, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Limatola, N.; Vasilev, F.; Chun, J.T.; Santella, L. Nicotine induces polyspermy in sea urchin eggs through a non-cholinergic pathway modulating actin dynamics. Cells 2020, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Limatola, N.; Chun, J.T.; Santella, L. Effects of Salinity and pH of seawater on the Reproduction of the Sea Urchin Paracentrotus lividus. Biol. Bull. 2020, 239, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Limatola, N.; Chun, J.T.; Santella, L. Regulation of the actin cytoskeleton-linked Ca2+ signaling by intracellular pH in fertilized eggs of sea urchin. Cells 2022, 11, 1496. [Google Scholar] [CrossRef]
- Limatola, N.; Chun, J.T.; Santella, L. Fertilization and development of Arbacia lixula eggs are affected by osmolality conditions. BioSystems 2021, 206, 104448. [Google Scholar] [CrossRef]
- Jaffe, L.A. Fast block to polyspermy in sea urchin eggs is electrically mediated. Nature 1976, 261, 68–71. [Google Scholar] [CrossRef]
- Wong, J.L.; Wessel, G.M. Major components of a sea urchin block to polyspermy are structurally and functionally conserved. Evol. Dev. 2004, 6, 134–153. [Google Scholar] [CrossRef]
- Vacquier, V.D.; Tegner, M.J.; Epel, D. Protease activity establishes the block against polysermy in sea urchin eggs. Nature 1972, 240, 352–353. [Google Scholar] [CrossRef]
- Just, E.E. The fertilization reaction in Echinarachnius parma. I. Cortical response of the egg to insemination. Biol. Bull. 1919, 36, 1–10. [Google Scholar] [CrossRef]
- Dale, B. New experimental data refuting the idea of a fast electrical block to polyspermy in sea urchin eggs. Zygote 2019, 27, 193–194. [Google Scholar] [CrossRef]
- Runft, L.; Jaffe, L.A.; Mehlmann, L.M. Egg Activation at Fertilization: Where It All Begins. Dev. Biol. 2002, 245, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Parrington, J.; Davis, L.C.; Galione, A.; Wessel, G. Flipping the switch: How a sperm activates the egg at fertilization. Dev. Dyn. 2007, 236, 2027–2038. [Google Scholar] [CrossRef] [PubMed]
- Santella, L.; Limatola, N.; Chun, J.T. Calcium and actin in the saga of awakening oocytes. Biochem. Biophys. Res. Commun. 2015, 460, 104–113. [Google Scholar] [CrossRef]
- Hirohashi, N.; Yanagimachi, R. Sperm acrosome reaction: Its site and role in fertilization. Biol. Reprod. 2018, 99, 127–133. [Google Scholar] [CrossRef]
- Hoshi, M.; Moriyama, H.; Matsumoto, M. Structure of acrosome reaction-inducing substance in the jelly coat of starfish eggs: A mini review. Biochem. Biophys. Res. Commun. 2012, 425, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Santella, L.; Vasilev, F.; Chun, J.T. Fertilization in echinoderms. Biochem. Biophys. Res. Commun. 2012, 425, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Dale, B.; Dan-Sohkawa, M.; De Santis, A.; Hoshi, M. Fertilization of the starfish Astropecten aurantiacus. Exp. Cell Res. 1981, 132, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Puppo, A.; Chun, J.T.; Gragnaniello, G.; Garante, E.; Santella, L. Alteration of the Cortical Actin Cytoskeleton Deregulates Ca2+ Signaling, Monospermic Fertilization, and Sperm Entry. PLoS ONE 2008, 3, e3588. [Google Scholar] [CrossRef]
- Santella, L.; Puppo, A.; Chun, J.T. The role of the actin cytoskeleton in calcium signaling in starfish oocytes. Int. J. Dev. Biol. 2008, 52, 571–584. [Google Scholar] [CrossRef]
- Santella, L.; Limatola, N.; Chun, J.T. Cellular and molecular aspects of oocyte maturation and fertilization: A perspective from the actin cytoskeleton. Zool. Lett. 2020, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Summers, R.G.; Hylander, B.L. Species-specificity of acrosome reaction and primary gamete binding in echinoids. Exp. Cell Res. 1975, 96, 63–68. [Google Scholar] [CrossRef]
- Epel, D. The program of fertilization. Sci. Am. 1977, 237, 128–139. [Google Scholar] [CrossRef]
- Metz, E.C.; Kane, R.E.; Yanagimachi, H.; Palumbi, S.R. Fertilization between closely related sea urchins is blocked by incompatibilities during sperm-egg attachment and early stages of fusion. Biol. Bull. 1994, 187, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Bleil, J.D.; Wassarman, P.M. Sperm-egg interactions in the mouse: Sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev. Biol. 1983, 95, 317–324. [Google Scholar] [CrossRef]
- Monroy, A.; Rosati, F.; Dale, B. Sperm-egg interaction. Boll. Zool. 1984, 51, 103–119. [Google Scholar] [CrossRef]
- Sawada, H.; Saito, T. Mechanisms of Sperm–Egg Interactions: What Ascidian Fertilization Research Has Taught Us. Cells 2022, 11, 2096. [Google Scholar] [CrossRef]
- Yanagimachi, R.; Cherr, G.; Matsubara, T.; Andoh, T.; Harumi, T.; Vines, C.; Pillai, M.; Griffin, F.; Matsubara, H.; Weatherby, T.; et al. Sperm attractant in the micropyle region of fish and insect eggs. Biol. Reprod. 2013, 88, 47, 1–11. [Google Scholar] [CrossRef]
- Dale, B.; Monroy, A. How is polyspermy prevented? Gamete Res. 1981, 4, 151–169. [Google Scholar] [CrossRef]
- Jin, M.; Fujiwara, E.; Kakiuchia, Y.; Okabe, M.; Satouh, Y.; Baba, S.A.; Chiba, K.; Hirohashi, N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 4892–4896. [Google Scholar] [CrossRef]
- Glabe, C.; Buchalter, M.; Lennarz, W.J. Studies on the interactions of sperm with the surface of the sea urchin egg. Dev. Biol. 1981, 84, 397–406. [Google Scholar] [CrossRef]
- Monroy, A.; Rosati, F. On the molecular mechanisms of sperm-egg interaction. Cell Differ. 1982, 11, 299–301. [Google Scholar] [CrossRef]
- Vacquier, V.D. The fertilizing capacity of sea urchin sperm rapidly decreases after induction of the acrosome reaction. Dev. Growth Differ. 1979, 21, 61–69. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limatola, N.; Chun, J.T.; Santella, L. Species-Specific Gamete Interaction during Sea Urchin Fertilization: Roles of the Egg Jelly and Vitelline Layer. Cells 2022, 11, 2984. https://doi.org/10.3390/cells11192984
Limatola N, Chun JT, Santella L. Species-Specific Gamete Interaction during Sea Urchin Fertilization: Roles of the Egg Jelly and Vitelline Layer. Cells. 2022; 11(19):2984. https://doi.org/10.3390/cells11192984
Chicago/Turabian StyleLimatola, Nunzia, Jong Tai Chun, and Luigia Santella. 2022. "Species-Specific Gamete Interaction during Sea Urchin Fertilization: Roles of the Egg Jelly and Vitelline Layer" Cells 11, no. 19: 2984. https://doi.org/10.3390/cells11192984