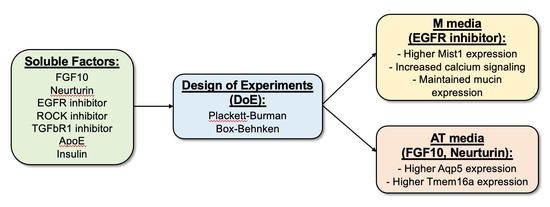
Optimizing Soluble Cues for Salivary Gland Tissue Mimetics Using a Design of Experiments (DoE) Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Animals
2.3. Primary Cell Isolation
2.4. Microbubble (MB) Array Fabrication
2.5. Cell Seeding
2.6. Media
2.7. Cell Viability
2.8. Plackett–Burman Design
2.9. Box–Behnken Design
2.10. RNA Extraction and qPCR
2.11. Calcium Signaling
2.12. Periodic Acid-Schiff’s Reagent-Alcian Blue (PAS-AB) Staining
2.13. Statistical Analysis
3. Results
3.1. Individual Factors Are Non-Toxic
3.2. FGF10, EGFR Inhibitor, ROCK Inhibitor, and Neurturin Increase Acinar Cell Relative mRNA Expression
3.3. The EGFR Inhibitor Promotes Mist1 mRNA Expression While FGF10 and Neurturin Promote Aqp5 and Tmem16a mRNA Expression
3.4. Model Validation Confirms Increases in Acinar Cell mRNA Expression in Mist1-Optimized and Aqp5/Tmem16a-Optimized Media
3.5. Addition of a ROCK Inhibitor for the First 24 h Had Minimal Impact on Mist1 mRNA Expression, but Decreased mRNA Expression of Duct and Myoepithelial Markers
3.6. Mist1-Promoting Media, Alone and in Combination with ROCK Inhibition for 24 h, Showed an Enhanced Calcium Signaling Response to Carbachol
3.7. Periodic Acid-Schiff’s Reagent/Alcian Blue (PAS-AB) Staining Showed Preservation of Mucin Expression in M and R24M Media
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Escobar, A.; Aitken-Saavedra, J.P. Xerostomia: An Update of Causes and Treatments. In Salivary Glands—New Approaches in Diagnostics and Treatment; IntechOpen: London, UK, 2019; pp. 15–37. [Google Scholar]
- Whelton, H. Introduction: Anatomy and Physiology of Salivary Glands. In Saliva and Oral Health; Stephen Hancock Limited: Orleton, UK, 2012; pp. 1–36. [Google Scholar]
- Wolff, A.; Joshi, R.K.; Ekstrom, J.; Aframian, D.; Pedersen, A.M.L.; Proctor, G.; Narayana, N.; Villa, A.; Sia, Y.W.; Aliko, A.; et al. A Guide to Medications Inducing Salivary Gland Dysfunction, Xerostomia, and Subjective Sialorrhea: A Systematic Review Sponsored by the World Workshop on Oral Medicine VI. Drugs R&D 2017, 17, 1–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Vissink, A.; Jansma, J.; Spijkervet, F.; Burlage, F.; Coppes, R. Oral Sequelae of Head and Neck Radiotherapy. Crit. Rev. Oral Biol. Med. 2003, 14, 199–212. [Google Scholar] [CrossRef]
- Kassan, S.S.; Moutsopoulos, H.M. Clinical Manifestations and Early Diagnosis of Sjögren Syndrome. Arch. Intern. Med. 2004, 164, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, H.; Baharvand, M.; Movahhedian, A.; Mohammadi, M.; Khodadoustan, A. Xerostomia Due to Systemic Disease: A Review of 20 Conditions and Mechanisms. Ann. Med. Health Sci. Res. 2014, 4, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Quissell, D.O.; Redman, R.S.; Mark, M.R. Short-Term Primary Culture of Acinar-Intercalated Duct Complexes from Rat Submandibular Glands. In Vitr. Cell. Dev. Biol. 1986, 22, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-K.; Mertz, P.M.; Oliver, C.; Baum, B.J.; Kousvelar, E.E. Cellular Characteristics of Long-Term Cultured Rat Parotid Acinar Cells. In Vitr. Cell. Dev. Biol. 1991, 27, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; Jones, J.P.; Lei, P.; Andreadis, S.T.; Baker, O.J. Laminin-111 Peptides Conjugated to Fibrin Hydrogels Promote Formation of Lumen Containing Parotid Gland Cell Clusters. Biomacromolecules 2016, 17, 2293–2301. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, Z.F.; Nelson, D.A.; Moskwa, N.; Sfakis, L.M.; Castracane, J.; Larsen, M. FGF2-Dependent Mesenchyme and Laminin-111 Are Niche Factors in Salivary Gland Organoids. J. Cell Sci. 2018, 131, jcs208728. [Google Scholar] [CrossRef] [Green Version]
- Vining, K.H.; Lombaert, I.M.A.; Patel, V.N.; Kibbey, S.E.; Pradhan-Bhatt, S.; Witt, R.L.; Hoffman, M.P. Neurturin-Containing Laminin Matrices Support Innervated Branching Epithelium from Adult Epithelial Salispheres. Biomaterials 2019, 216, 118245. [Google Scholar] [CrossRef]
- Song, Y.; Uchida, H.; Sharipol, A.; Piraino, L.; Mereness, J.A.; Ingalls, M.H.; Rebhahn, J.; Newlands, S.D.; DeLouise, L.A.; Ovitt, C.E.; et al. Development of a Functional Salivary Gland Tissue Chip with Potential for High-Content Drug Screening. Commun. Biol. 2021, 4, 1–15. [Google Scholar] [CrossRef]
- Miyajima, H.; Matsumoto, T.; Sakai, T.; Yamaguchi, S.; An, S.H.; Abe, M.; Wakisaka, S.; Lee, K.Y.; Egusa, H.; Imazato, S. Hydrogel-Based Biomimetic Environment for in Vitro Modulation of Branching Morphogenesis. Biomaterials 2011, 32, 6754–6763. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Inaba, T.; Murakami-Sekimata, A.; Nogawa, H. Morphogenesis and Mucus Production of Epithelial Tissues of Three Major Salivary Glands of Embryonic Mouse in 3D Culture. Zoolog. Sci. 2017, 34, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, D.; Pinto, F.; Senoo, M. Inhibition of TGF-b Signaling Supports High Proliferative Potential of Diverse P63+ Mouse Epithelial Progenitor Cells In Vitro. Sci. Rep. 2017, 7, 6089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; An, G.H.; Woo, D.-H.; Kim, J.-H.; Park, H.-K. Rho-Associated Kinase Inhibitor Enhances the Culture Condition of Isolated Mouse Salivary Gland Cells in Vitro. Tissue Cell 2018, 54, 20–25. [Google Scholar] [CrossRef]
- Koslow, M.; O’Keefe, K.J.; Hosseini, Z.F.; Nelson, D.A.; Larsen, M. ROCK Inhibitor Increases Proacinar Cells in Adult Salivary Gland Organoids. Stem Cell Res. 2019, 41, 101608. [Google Scholar] [CrossRef]
- Sui, Y.; Zhang, S.; Li, Y.; Zhang, X.; Hu, W.; Feng, Y.; Xiong, J.; Zhang, Y.; Wei, S. Generation of Functional Salivary Gland Tissue from Human Submandibular Gland Stem/Progenitor Cells. Stem Cell Res. Ther. 2020, 11, 127. [Google Scholar] [CrossRef] [Green Version]
- Funkenbusch, P. Array Design (Two-Level Factors). In Practical Guide to Designed Experiments, A Unified Modular Approach; Marcel Dekker: New York, NY, USA, 2005; pp. 55–88. [Google Scholar]
- Pin, C.L.; Rukstalis, J.M.; Johnson, C.; Konieczny, S.F. The BHLH Transcription Factor Mist1 Is Required to Maintain Exocrine Pancreas Cell Organization and Acinar Cell Identity. J. Cell Biol. 2001, 155, 519–530. [Google Scholar] [CrossRef] [Green Version]
- Larsen, H.S.; Aure, M.H.; Peters, S.B.; Larsen, M.; Messelt, E.B.; Galtung, H.K. Localization of AQP5 during Development of the Mouse Submandibular Salivary Gland. J. Mol. Histol. 2011, 42, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Romanenko, V.G.; Catalan, M.A.; Brown, D.A.; Putzier, I.; Hartzell, H.C.; Marmorstein, A.D.; Gonzalez-Begne, M.; Rock, J.R.; Harfe, B.D.; Melvin, J.E. Tmem16A Encodes the Ca2+-Activated Cl− Channel in Mouse Submandibular Salivary Gland Acinar Cells. J. Biol. Chem. 2010, 285, 12990–13001. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Min, S.; Kim, D.; Kim, H.; Roh, S. A Rho Kinase (ROCK) Inhibitor, Y-27632, Inhibits the Dissociation-Induced Cell Death of Salivary Gland Stem Cells. Molecules 2021, 26, 2658. [Google Scholar] [CrossRef] [PubMed]
- Means, A.L.; Meszoely, I.M.; Suzuki, K.; Miyamoto, Y.; Rustgi, A.K.; Coffey, R.J.; Wright, C.V.E.; Stoffers, D.A.; Leach, S.D. Pancreatic Epithelial Plasticity Mediated by Acinar Cell Transdifferentiation and Generation of Nestin-Positive Intermediates. Dev. Dis. 2005, 132, 3767–3776. [Google Scholar] [CrossRef] [Green Version]
- Janebodin, K.; Buranaphatthana, W.; Ieronimakis, N.; Hays, A.L.; Reyes, M. An In Vitro Culture System for Long-Term Expansion of Epithelial and Mesenchymal Salivary Gland Cells: Role of TGF-B1 in Salivary Gland Epithelial and Mesenchymal Differentiation. BioMed Res. Int. 2013, 2013, 815895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quissell, D.; Redman, R.; Barzen, K.; McNutt, R. Effects of Oxygen, Insulin, and Glucagon Concentrations on Rat Submandibular Acini in Serum-Free Primary Culture. In Vitr. Cell. Dev. Biol.-Anim. 1994, 30, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.I.; Galdos, F.X.; Dinan, K.A.; Jedrychowski, M.P.; Davis, J.C.; Vujic, A.; Rachmin, I.; Shigley, C.; Pancoast, J.R.; Lee, S.; et al. Apolipoprotein E Is a Pancreatic Extracellular Factor That Maintains Mature β-Cell Gene Expression. PLoS ONE 2018, 13, e0204595. [Google Scholar] [CrossRef]
- Steinberg, Z.; Myers, C.; Heim, V.M.; Lathrop, C.A.; Rebustini, I.T.; Stewart, J.S.; Larsen, M.; Hoffman, M.P. FGFR2b Signaling Regulates Ex Vivo Submandibular Gland Epithelial Cell Proliferation and Branching Morphogenesis. Development 2005, 132, 1223–1234. [Google Scholar] [CrossRef] [Green Version]
- Pinkstaff, C.A. Salivary Gland Sexual Dimorphism: A Brief Review. Eur. J. Morphol. 1998, 36, 31–34. [Google Scholar]
- Maruyama, C.L.; Monroe, M.; Hunt, J.; Buchman, L.; Baker, O.J. Comparing Human and Mouse Salivary Glands: A Practice Guide for Salivary Researchers. Oral Dis. 2019, 25, 403–415. [Google Scholar] [CrossRef]
- Song, Y.; Sharipol, A.; Uchida, H.; Ingalls, M.H.; Piraino, L.; Mereness, J.A.; Moyston, T.; DeLouise, L.A.; Ovitt, C.E.; Benoit, D.S.W. Encapsulation of Primary Salivary Gland Acinar Cell Clusters and Intercalated Ducts (AIDUCs) within Matrix Metalloproteinase (MMP)-Degradable Hydrogels to Maintain Tissue Structure and Function. Adv. Healthc. Mater. 2022, 11, 2101948. [Google Scholar] [CrossRef]
- Giang, U.-B.T.; Lee, D.; King, M.R.; DeLouise, L.A. Microfabrication of Cavities in Polydimethylsiloxane Using DRIE Silicon Molds. Lab Chip 2007, 7, 1660–1662. [Google Scholar] [CrossRef] [Green Version]
- Fairbanks, B.D.; Schwartz, M.P.; Bowman, C.N.; Anseth, K.S. Photoinitiated Polymerization of PEG-Diacrylate with Lithium Phenyl-2,4,6-Trimethylbenzoylphosphinate: Polymerization Rate and Cytocompatibility. Biomaterials 2009, 30, 6702–6707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shubin, A.D.; Felong, T.J.; Graunke, D.; Ovitt, C.E.; Benoit, D.S.W. Development of Poly (Ethylene Glycol) Hydrogels for Salivary Gland Tissue Engineering Applications. Tissue Eng. Part A 2015, 21, 1733–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puente-Massaguer, E.; Badiella, L.; Gutierrez-Granados, S.; Cervera, L.; Godia, F. A Statistical Approach to Improve Compound Screening in Cell Culture Media. Eng. Life Sci. 2019, 19, 315–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, C.; Rothermel, A.; Robitzki, A.A. Neurturin, a Member of the Glial Cell Line-Derived Neurotrophic Factor Family, Affects the Development of Acetylcholinesterase-Positive Cells in a Three-Dimensional Model System of Retinogenesis. J. Neurochem. 2008, 107, 96–104. [Google Scholar] [CrossRef]
- Chandra, A.; Angle, N. Vascular Endothelial Growth Factor Stimulates a Novel Calcium-Signaling Pathway in Vascular Smooth Muscle Cells. Surgery 2005, 138, 780–787. [Google Scholar] [CrossRef]
- Chausson, P.; Leresche, N.; Lambert, R.C. Dynamics of Intrinsic Dendritic Calcium Signaling during Tonic Firing of Thalamic Reticular Neurons. PLoS ONE 2013, 8, e72275. [Google Scholar] [CrossRef] [Green Version]
- Price, L.S.; Langeslag, M.; ten Klooster, J.P.; Hordijk, P.L.; Jalink, K.; Collard, J.G. Calcium Signaling Regulates Translocation and Activation of Rac. J. Biol. Chem. 2003, 278, P39413–P39421. [Google Scholar] [CrossRef] [Green Version]
- Tao, X.; Chen, Q.; Li, N.; Xiang, H.; Pan, Y.; Qu, Y.; Shang, D.; Go, V.L.W.; Xue, J.; Sun, Y.; et al. Serotonin-RhoA/ROCK Axis Promotes Acinar-to-Ductal Metaplasia in T Caerulein-Induced Chronic Pancreatitis. Biomed. Pharmacother. 2020, 125, 109999. [Google Scholar] [CrossRef]
- Zhang, L.; Valdez, J.M.; Zhang, B.; Wei, L.; Chang, J.; Xin, L. ROCK Inhibitor Y-27632 Suppresses Dissociation-Induced Apoptosis of Murine Prostate Stem/Progenitor Cells and Increases Their Cloning Efficiency. PLoS ONE 2011, 6, e18271. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; Zhang, Y. Establishment of Long-Term Serum-Free Culture for Lacrimal Gland Stem Cells Aiming at Lacrimal Gland Repair. Stem Cell Res. Ther. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Lee, H.-W.; Hsiao, Y.-C.; Young, T.-H.; Yang, T.-L. Maintenance of the Spheroid Organization and Properties of Glandular Progenitor Cells by Fabricated Chitosan Based Biomaterials. Biomater. Sci. 2018, 6, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Shubin, A.D.; Sharipol, A.; Felong, T.J.; Weng, P.-L.; Schutrum, B.E.; Joe, D.S.; Aure, M.H.; Benoit, D.S.W.; Ovitt, C.E. Stress or Injury Induces Cellular Plasticity in Salivary Gland Acinar Cells. Cell Tissue Res. 2020, 380, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Wanatabe, H.; Takahashi, H.; Hata-Kawakami, M.; Tanaka, A. Expression of C-Kit and Cytokeratin 5 in the Submandibular Gland after Release of Long-Term Ligation of the Main Excretory Duct in Mice. Acta Histochem. Cytochem. 2017, 50, 111–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambudkar, I.S. Calcium Signalling in Salivary Gland Physiology and Dysfunction. J. Physiol. 2016, 594, 2813–2824. [Google Scholar] [CrossRef] [Green Version]
- Takano, T.; Wahl, A.M.; Huang, K.-T.; Narita, T.; Rugis, J.; Sneyd, J.; Yule, D.I. The Characteristics of Intracellular Ca2+ Signals in Vivo Necessitate a New Model for Salivary Fluid Secretion. eLife 2021, 10, e66170. [Google Scholar] [CrossRef]
- Gilman, K.E.; Camden, J.M.; Klein, R.R.; Zhang, Q.; Weisman, G.A.; Limesand, K.H. P2X7 Receptor Deletion Suppresses γ-Radiation-Induced Hyposalivation. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2019, 316, R687–R696. [Google Scholar] [CrossRef]
- Tabak, L.A. Structure and Function of Human Salivary Mucins. Crit. Rev. Oral Biol. Med. 1990, 1, 229–234. [Google Scholar] [CrossRef]
- Gaber, W.; Shalaan, S.A.; Misk, N.A.; Ibrahim, A. Surgical Anatomy, Morphometry, and Histochemistry of Major Salivary Glands in Dogs: Updates and Recommendations. Int. J. Vet. Health Sci. Res. 2020, 8, 252–259. [Google Scholar]
- Zhang, X.-M.; Huang, Y.; Zhang, K.; Qu, L.-H.; Cong, X.; Su, J.-Z.; Wu, L.-L.; Yu, G.-Y.; Zhang, Y. Expression Patterns of Tight Junction Proteins in Porcine Major Salivary Glands: A Comparison Study with Human and Murine Glands. J. Anat. 2018, 233, 167–176. [Google Scholar] [CrossRef]
- Wang, J.-K.; Chiu, H.-H.; Hsieh, C.-S. Optimization of the Medium Components by Statistical Experimental Methods to Enhance Nattokinase Activity. Fooyin J. Health Sci. 2009, 1, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Haque, S.; Niwas, R.; Srivastava, A.; Pasupuleti, M.; Tripathi, C.K.M. Strategies for Fermentation Medium Optimization: An In-Depth Review. Front. Microbiol. 2017, 7, 2087. [Google Scholar] [CrossRef] [PubMed]
- Singleton, C.; Gilman, J.; Rollit, J.; Zhang, K.; Parker, D.A.; Love, J. A Design of Experiments Approach for the Rapid Formulation of a Chemically Defined Medium for Metabolic Profiling of Industrially Important Microbes. PLoS ONE 2019, 14, e0218208. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, A.; Finley, J.K.; Knox, S.M. Salivary Gland Development and Disease. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 573–590. [Google Scholar] [CrossRef] [PubMed]
- Knox, S.M.; Lombaert, M.A.; Reed, X.; Vitale-Cross, L.; Gutkind, J.S.; Hoffman, M.P. Parasympathetic Innervation Maintains Epithelial Progenitor Cells During Salivary Organogenesis. Science 2010, 329, 1645–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Tran, T.; Rukstalis, J.M.; Sun, P.; Damsz, B.; Konieczny, S.F. Inhibition of Mist1 Homodimer Formation Induces Pancreatic Acinar-to-Ductal Metaplasia. Mol. Cell. Biol. 2004, 24, 2673–2681. [Google Scholar] [CrossRef] [Green Version]
- Karki, A.; Humphrey, S.E.; Steele, R.E.; Hess, D.A.; Taparowsky, E.J.; Konieczny, S.F. Silencing Mist1 Gene Expression Is Essential for Recovery from Acute Pancreatitis. PLoS ONE 2015, 10, e0145724. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.; DiRenzo, D.; Qu, C.; Barney, D.; Miley, D.; Konieczny, S.F. Maintenance of Acinar Cell Organization Is Critical to Preventing Kras-Induced Acinar-Ductal Metaplasia. Oncogene 2013, 32, 1950–1958. [Google Scholar] [CrossRef] [Green Version]
- Storz, P. Acinar Cell Plasticity and Development of Pancreatic Ductal Adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 296–304. [Google Scholar] [CrossRef]
- Hauser, B.R.; Aure, M.H.; Kelly, M.C.; Hoffman, M.P.; Chibly, A.M. Generation of a Single-Cell RNAseq Atlas of Murine Salivary Gland Development. Iscience 2020, 23, 101838. [Google Scholar] [CrossRef]
- Shubin, A.D.; Felong, T.J.; Schutrum, B.E.; Joe, D.S.L.; Ovitt, C.E.; Benoit, D.S.W. Encapsulation of Primary Salivary Gland Cells in Enzymatically Degradable Poly (Ethylene Glycol) Hydrogels Promotes Acinar Cell Characteristics. Acta Biomater. 2017, 50, 437–449. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, K.S.; Zeeberg, K.; Sauter, D.R.P.; Poulsen, K.A.; Hoffman, E.K.; Schwab, A. The Role of TMEM16A (ANO1) and TMEM16F (ANO6) in Cell Migration. Pflüg. Arch.-Eur. J. Physiol. 2013, 465, 1753–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmerson, E.; May, A.J.; Nathan, S.; Cruz-Pacheo, N.; Lizama, C.O.; Maliskova, L.; Zovein, A.C.; Shen, Y.; Muench, M.O.; Knox, S.M. SOX2 Regulates Acinar Cell Development in the Salivary Gland. eLife 2017, 6, e26620. [Google Scholar] [CrossRef] [PubMed]
- Spandidos, A.; Wang, X.; Wang, H.; Seed, B. PrimerBank: A Resource of Human and Mouse PCR Primer Pairs for Gene Expression Detection and Quantification. Nucleic Acids Res. 2010, 38, D792–D799. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Plackett–Burman | |||
|---|---|---|---|
| Factor | Low Level (−1) | High Level (+1) | Reference |
| Neurturin | 0 | 1 ng/mL | [12] |
| TGFβR1 inhibitor | 0 | 1 µM | [16,26] |
| EGFR inhibitor | 0 | 0.5 µM | [11,25] |
| ROCK inhibitor | 0 | 10 µM | [17,18,24] |
| Apolipoprotein E | 0 | 1 µg/mL | [28] |
| FGF10 | 0 | 0.1 µg/mL | [19,29] |
| Insulin | 0.03 µg/mL | 10 µg/mL | [27] |
| Box–Behnken | |||
| Factor | Low Level (−1) | Middle Level (0) | High Level (+1) |
| Neurturin | 0 | 5 ng/mL | 10 ng/mL |
| EGFR inhibitor | 0 | 0.25 µM | 0.5 µM |
| FGF10 | 0 | 0.25 µg/mL | 0.5 µg/mL |
| Factor | |||||||
|---|---|---|---|---|---|---|---|
| Run # | Fgf10 | EGFR Inhibitor | TGFβR1 Inhibitor | ROCK Inhibitor | Neurturin | Apolipoprotein E | Insulin |
| 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 |
| 2 | −1 | −1 | 1 | −1 | −1 | 1 | −1 |
| 3 | 1 | 1 | 1 | −1 | −1 | −1 | 1 |
| 4 | 1 | −1 | 1 | 1 | 1 | −1 | −1 |
| 5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 6 | −1 | −1 | −1 | 1 | −1 | −1 | 1 |
| 7 | −1 | 1 | −1 | −1 | 1 | −1 | 1 |
| 8 | −1 | −1 | 1 | −1 | 1 | 1 | 1 |
| 9 | −1 | 1 | −1 | 1 | 1 | 1 | −1 |
| 10 | −1 | 1 | 1 | 1 | −1 | −1 | −1 |
| 11 | 1 | −1 | −1 | −1 | 1 | −1 | −1 |
| 12 | 1 | −1 | −1 | 1 | −1 | 1 | 1 |
| 13 | −1 | −1 | 1 | 1 | 1 | −1 | 1 |
| 14 | 1 | 1 | −1 | 1 | 1 | −1 | 1 |
| 15 | −1 | −1 | −1 | 1 | 1 | 1 | −1 |
| 16 | −1 | 1 | −1 | −1 | −1 | 1 | 1 |
| 17 | −1 | −1 | −1 | −1 | −1 | −1 | −1 |
| 18 | 1 | 1 | 1 | −1 | 1 | 1 | −1 |
| 19 | 1 | −1 | 1 | 1 | −1 | 1 | −1 |
| 20 | 1 | 1 | −1 | 1 | −1 | −1 | −1 |
| 21 | 1 | −1 | 1 | −1 | −1 | −1 | 1 |
| 22 | 1 | −1 | −1 | −1 | 1 | 1 | 1 |
| 23 | −1 | 1 | 1 | 1 | −1 | 1 | 1 |
| 24 | −1 | 1 | 1 | −1 | 1 | −1 | −1 |
| Factor | |||
|---|---|---|---|
| Run # | EGFR Inhibitor | FGF10 | Neurturin |
| 1 | 1 | 1 | 0 |
| 2 | −1 | 0 | −1 |
| 3 | 1 | 0 | −1 |
| 4 | −1 | 1 | 0 |
| 5 | 0 | −1 | 1 |
| 6 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 |
| 8 | 1 | −1 | 0 |
| 9 | −1 | 0 | 1 |
| 10 | 0 | −1 | −1 |
| 11 | 0 | 1 | 1 |
| 12 | 0 | 0 | 0 |
| 13 | 0 | 1 | −1 |
| 14 | 1 | 0 | 1 |
| 15 | −1 | −1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piraino, L.R.; Benoit, D.S.W.; DeLouise, L.A. Optimizing Soluble Cues for Salivary Gland Tissue Mimetics Using a Design of Experiments (DoE) Approach. Cells 2022, 11, 1962. https://doi.org/10.3390/cells11121962
Piraino LR, Benoit DSW, DeLouise LA. Optimizing Soluble Cues for Salivary Gland Tissue Mimetics Using a Design of Experiments (DoE) Approach. Cells. 2022; 11(12):1962. https://doi.org/10.3390/cells11121962
Chicago/Turabian StylePiraino, Lindsay R., Danielle S. W. Benoit, and Lisa A. DeLouise. 2022. "Optimizing Soluble Cues for Salivary Gland Tissue Mimetics Using a Design of Experiments (DoE) Approach" Cells 11, no. 12: 1962. https://doi.org/10.3390/cells11121962