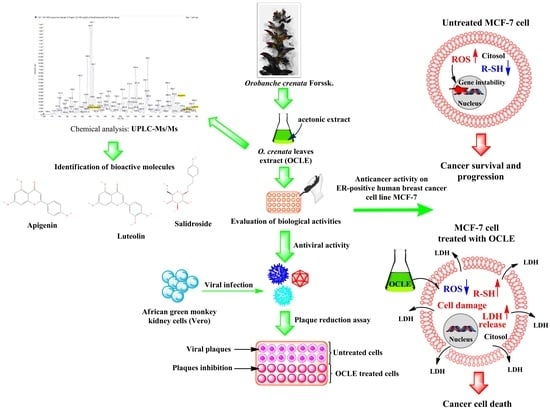
Orobanche crenata Forssk. Extract Affects Human Breast Cancer Cell MCF-7 Survival and Viral Replication
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Preparation of OCLE Extract
2.3. Antitumor Activity
2.3.1. Cell Culture
2.3.2. MTT Assay
2.3.3. Lactic Dehydrogenase Release
2.3.4. Intracellular Reactive Oxygen Species Assay
2.3.5. Thiol Group Determination
2.4. Antioxidant Activity
2.4.1. DPPH Assay
2.4.2. ABTS Assay
2.5. Antiviral Activity
2.5.1. Cell Viability
2.5.2. Antiviral Activity of OCLE
2.5.3. Plaque Reduction Assay
2.5.4. Cytopathic Effect Inhibition Assays
2.6. Chemical Profile of OCLE
2.7. Statistical Analysis
3. Results
3.1. Antitumor Activity of OCLE
3.1.1. MTT Assay
3.1.2. Lactic Dehydrogenase Release
3.2. Antioxidant Activity of OCLE
3.2.1. Reactive Oxygen Species Levels
3.2.2. Thiol Group Determination
3.2.3. DPPH and ABTS Assays
3.3. Antiviral Activity of OCLE
Cell Viability
3.4. Chemical Profile of OCLE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Katsura, C.; Ogunmwonyi, I.; Kankam, H.K.; Saha, S. Breast cancer: Presentation, investigation and management. Br. J. Hosp. Med. 2022, 83, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Trogdon, J.G.; Baggett, C.D.; Gogate, A.; Reeder-Hayes, K.E.; Rotter, J.; Zhou, X.; Ekwueme, D.U.; Fairley, T.L.; Wheeler, S.B. Medical costs associated with metastatic breast cancer in younger, midlife, and older women. Breast Cancer Res. Treat. 2020, 181, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Trayes, K.P.; Cokenakes, S.E.H. Breast Cancer Treatment. Am. Fam. Physician 2021, 104, 171–178. [Google Scholar] [PubMed]
- Turner, K.M.; Yeo, S.K.; Holm, T.M.; Shaughnessy, E.; Guan, J.-L. Heterogeneity within molecular subtypes of breast cancer. Am. J. Physiol. Physiol. 2021, 321, C343–C354. [Google Scholar] [CrossRef] [PubMed]
- Parise, C.A.; Bauer, K.R.; Brown, M.M.; Caggiano, V. Breast Cancer Subtypes as Defined by the Estrogen Receptor (ER), Progesterone Receptor (PR), and the Human Epidermal Growth Factor Receptor 2 (HER2) among Women with Invasive Breast Cancer in California, 1999–2004. Breast J. 2009, 15, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Rasha, F.; Sharma, M.; Pruitt, K. Mechanisms of endocrine therapy resistance in breast cancer. Mol. Cell. Endocrinol. 2021, 532, 111322. [Google Scholar] [CrossRef]
- Ribi, K.; Luo, W.; Bernhard, J.; Francis, P.; Burstein, H.J.; Ciruelos, E.; Bellet, M.; Pavesi, L.; Lluch, A.; Visini, M.; et al. Adjuvant Tamoxifen Plus Ovarian Function Suppression Versus Tamoxifen Alone in Premenopausal Women with Early Breast Cancer: Patient-Reported Outcomes in the Suppression of Ovarian Function Trial. J. Clin. Oncol. 2016, 34, 1601–1610. [Google Scholar] [CrossRef]
- Murphy, C.C.; Bartholomew, L.K.; Carpentier, M.Y.; Bluethmann, S.M.; Vernon, S.W. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: A systematic review. Breast Cancer Res. Treat. 2012, 134, 459–478. [Google Scholar] [CrossRef] [Green Version]
- Vaughn, J.E.; Ammermann, C.; Lustberg, M.B.; Bickel, W.K.; Stein, J.S. Delay discounting and adjuvant endocrine therapy adherence in hormone receptor-positive breast cancer. Health Psychol. 2021, 40, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Montagna, E.; Zagami, P.; Masiero, M.; Mazzocco, K.; Pravettoni, G.; Munzone, E. Assessing Predictors of Tamoxifen Nonadherence in Patients with Early Breast Cancer. Patient Prefer. Adherence 2021, 15, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- Pons-Salort, M.; Grassly, N.C. Serotype-specific immunity explains the incidence of diseases caused by human enteroviruses. Science 2018, 361, 800–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lion, T. Adenovirus Infections in Immunocompetent and Immunocompromised Patients. Clin. Microbiol. Rev. 2014, 27, 441–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borchers, A.T.; Chang, C.; Gershwin, M.E.; Gershwin, L.J. Respiratory Syncytial Virus—A Comprehensive Review. Clin. Rev. Allergy Immunol. 2013, 45, 331–379. [Google Scholar] [CrossRef] [PubMed]
- Rechenchoski, D.Z.; Faccin-Galhardi, L.C.; Linhares, R.E.C.; Nozawa, C. Herpesvirus: An underestimated virus. Folia Microbiol. 2017, 62, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Iadevaia, C.; Perrotta, F.; Mazzeo, G.; Cerqua, F.S.; Mazzarella, G.; Guarino, S.; Parrella, R.; Bianco, A. Incidental diagnosis of lung adenocarcinoma following coronavirus OC 43 severe pneumonia. Monaldi Arch. Chest Dis. 2020, 90, 425–427. [Google Scholar] [CrossRef]
- Tompa, D.R.; Immanuel, A.; Srikanth, S.; Kadhirvel, S. Trends and strategies to combat viral infections: A review on FDA approved antiviral drugs. Int. J. Biol. Macromol. 2021, 172, 524–541. [Google Scholar] [CrossRef]
- D’Angeli, F.; Malfa, G.; Garozzo, A.; Volti, G.L.; Genovese, C.; Stivala, A.; Nicolosi, D.; Attanasio, F.; Bellia, F.; Ronsisvalle, S.; et al. Antimicrobial, Antioxidant, and Cytotoxic Activities of Juglans regia L. Pellicle Extract. Antibiotics 2021, 10, 159. [Google Scholar] [CrossRef]
- Genovese, C.; Cambria, M.T.; D’Angeli, F.; Addamo, A.P.; Malfa, G.A.; Siracusa, L.; Pulvirenti, L.; Anfuso, C.D.; Lupo, G.; Salmeri, M. The double effect of walnut septum extract (Juglans regia L.) counteracts A172 glioblastoma cell survival and bacterial growth. Int. J. Oncol. 2020, 57, 1129–1144. [Google Scholar] [CrossRef]
- Taviano, M.F.; Miceli, N.; Acquaviva, R.; Malfa, G.A.; Ragusa, S.; Giordano, D.; Cásedas, G.; Les, F.; López, V. Cytotoxic, Antioxidant, and Enzyme Inhibitory Properties of the Traditional Medicinal Plant Matthiola incana (L.) R. Br. Biology 2020, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, R.; D’Angeli, F.; Malfa, G.A.; Ronsisvalle, S.; Garozzo, A.; Stivala, A.; Ragusa, S.; Nicolosi, D.; Salmeri, M.; Genovese, C. Antibacterial and anti-biofilm activities of walnut pellicle extract (Juglans regia L.) against coagulase-negative staphylococci. Nat. Prod. Res. 2021, 35, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.N.M.; Saeed, N.A.-H.A.A.H.; Omear, H.A. The Anticancer Properties of Artemisia aucheri Boiss Extract on HT29 Colon Cancer Cells. J. Gastrointest. Cancer 2020, 52, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kurz, H.; Karygianni, L.; Argyropoulou, A.; Hellwig, E.; Skaltsounis, A.L.; Wittmer, A.; Vach, K.; Al-Ahmad, A. Antimicrobial Effects of Inula viscosa Extract on the In Situ Initial Oral Biofilm. Nutrients 2021, 13, 4029. [Google Scholar] [CrossRef] [PubMed]
- Genovese, C.; D’Angeli, F.; Bellia, F.; Distefano, A.; Spampinato, M.; Attanasio, F.; Nicolosi, D.; Di Salvatore, V.; Tempera, G.; Lo Furno, D. In Vitro Antibacterial, Anti-Adhesive and Anti-Biofilm Activities of Krameria lappacea (Dombey) Burdet & BB Simpson Root Extract against Methicillin-Resistant Staphylococcus aureus Strains. Antibiotics 2021, 10, 428. [Google Scholar] [PubMed]
- Acquaviva, R.; Genovese, C.; Amodeo, A.; Tomasello, B.; Malfa, G.; Sorrenti, V.; Tempera, G.; Addamo, A.P.; Ragusa, S.; Rosa, T. Biological activities of Teucrium flavum L., Teucrium fruticans L., and Teucrium siculum rafin crude extracts. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2018, 152, 720–727. [Google Scholar] [CrossRef]
- Nayim, P.; Sudhir, K.; Mbaveng, A.T.; Kuete, V.; Sanjukta, M. In Vitro Anticancer Activity of Imperata cylindrica Root’s Extract toward Human Cervical Cancer and Identification of Potential Bioactive Compounds. BioMed Res. Int. 2021, 2021, 4259777. [Google Scholar] [CrossRef]
- Behl, T.; Rocchetti, G.; Chadha, S.; Zengin, G.; Bungau, S.; Kumar, A.; Mehta, V.; Uddin, S.; Khullar, G.; Setia, D.; et al. Phytochemicals from Plant Foods as Potential Source of Antiviral Agents: An Overview. Pharmaceuticals 2021, 14, 381. [Google Scholar] [CrossRef]
- Domina, G.; Marino, P.; Castellano, G. The genus Orobanche (Orobanchaceae) in Sicily. Flora Medit. 2011, 21, 205–242. [Google Scholar]
- Genovese, C.; D’Angeli, F.; Attanasio, F.; Caserta, G.; Scarpaci, K.S.; Nicolosi, D. Phytochemical composition and biological activities of Orobanche crenata Forssk.: A review. Nat. Prod. Res. 2020, 35, 4579–4595. [Google Scholar] [CrossRef]
- Rubiales, D.; Heide-Jørgensen, H.S. Parasitic Plants; Wiley Online Library: Hoboken, NJ, USA, 2011. [Google Scholar]
- Heinrich, M.; Leonti, M.; Nebel, S.; Peschel, W.; Pieroni, A.; Smith, F. Understanding local Mediterranean diets: A multidisciplinary pharmacological and ethnobotanical approach. Pharmacol. Res. 2005, 52, 353–366. [Google Scholar] [CrossRef]
- Genovese, C.; Acquaviva, R.; Ronsisvalle, S.; Tempera, G.; Malfa, G.A.; D’Angeli, F.; Ragusa, S.; Nicolosi, D. In vitro evaluation of biological activities of Orobanche crenata Forssk. leaves extract. Nat. Prod. Res. 2020, 34, 3234–3238. [Google Scholar] [CrossRef] [PubMed]
- D’Angeli, F.; Guadagni, F.; Genovese, C.; Nicolosi, D.; Salinaro, A.T.; Spampinato, M.; Mannino, G.; Furno, D.L.; Petronio, G.P.; Ronsisvalle, S.; et al. Anti-Candidal Activity of the Parasitic Plant Orobanche crenata Forssk. Antibiotics 2021, 10, 1373. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.G.; Imam, A.M.; Abdelghany, B.E. Evaluation of cytotoxic and anticancer effect of Orobanche crenata methanolic extract on cancer cell lines. Tumor Biol. 2020, 42. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, B.; Di Mauro, M.D.; Malfa, G.A.; Acquaviva, R.; Sinatra, F.; Spampinato, G.; Laudani, S.; Villaggio, G.; Bielak-Zmijewska, A.; Grabowska, W.; et al. Rapha Myr®, a Blend of Sulforaphane and Myrosinase, Exerts Antitumor and Anoikis-Sensitizing Effects on Human Astrocytoma Cells Modulating Sirtuins and DNA Methylation. Int. J. Mol. Sci. 2020, 21, 5328. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Cutrí, C.C.; Garozzo, A.; Siracusa, M.A.; Sarvá, M.C.; Tempera, G.; Geremia, E.; Pinizzotto, M.R.; Guerrera, F. Synthesis and antiviral activity of a new series of 4-isothiazolecarbonitriles. Bioorg. Med. Chem. 1998, 6, 2271–2280. [Google Scholar] [CrossRef]
- Song, X.; He, J.; Xu, H.; Hu, X.-P.; Wu, X.-L.; Wu, H.-Q.; Liu, L.-Z.; Liao, C.-H.; Zeng, Y.; Li, Y.; et al. The antiviral effects of acteoside and the underlying IFN-γ-inducing action. Food Funct. 2016, 7, 3017–3030. [Google Scholar] [CrossRef] [Green Version]
- Martins, F.O.; Esteves, P.F.; Mendes, G.; Barbi, N.S.; Menezes, F.S.; Romanos, M.T. Verbascoside isolated from Lepechinia speciosa has inhibitory Activity against HSV-1 and HSV-2 in vitro. Nat. Prod. Commun. 2009, 4, 1693–1696. [Google Scholar] [CrossRef] [Green Version]
- Şenol, H.; Tulay, P.; Ergören, M.C.; Hanoğlu, A.; Çaliş, I.; Mocan, G. Cytotoxic Effects of Verbascoside on MCF-7 and MDA-MB-231. Turk. J. Pharm. Sci. 2021, 18, 637–644. [Google Scholar] [CrossRef]
- Vasincu, A.; Neophytou, C.M.; Luca, S.V.; Skalicka-Woźniak, K.; Miron, A.; Constantinou, A.I. 6-O-(3″, 4″-di-O-trans-cinnamoyl)-α-l-rhamnopyranosylcatalpol and verbascoside: Cytotoxicity, cell cycle kinetics, apoptosis, and ROS production evaluation in tumor cells. J. Biochem. Mol. Toxicol. 2020, 34, e22443. [Google Scholar] [CrossRef] [PubMed]
- D’Imperio, M.; Cardinali, A.; D’Antuono, I.; Linsalata, V.; Minervini, F.; Redan, B.; Ferruzzi, M. Stability–activity of verbascoside, a known antioxidant compound, at different pH conditions. Food Res. Int. 2014, 66, 373–378. [Google Scholar] [CrossRef]
- Qian, S.; Fan, W.; Qian, P.; Zhang, D.; Wei, Y.; Chen, H.; Li, X. Apigenin Restricts FMDV Infection and Inhibits Viral IRES Driven Translational Activity. Viruses 2015, 7, 1613–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korga-Plewko, A.; Michalczyk, M.; Adamczuk, G.; Humeniuk, E.; Ostrowska-Lesko, M.; Jozefczyk, A.; Iwan, M.; Wojcik, M.; Dudka, J. Apigenin and Hesperidin Downregulate DNA Repair Genes in MCF-7 Breast Cancer Cells and Augment Doxorubicin Toxicity. Molecules 2020, 25, 4421. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jung, J.; Jeong, N.Y.; Chung, H.-J. The natural plant flavonoid apigenin is a strong antioxidant that effectively delays peripheral neurodegenerative processes. Anat. Sci. Int. 2019, 94, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Sosic, A.; Cappellini, M.; Sinigaglia, L.; Jacquet, R.; Deffieux, D.; Fabris, D.; Quideau, S.; Gatto, B. Polyphenolic C-glucosidic ellagitannins present in oak-aged wine inhibit HIV-1 nucleocapsid protein. Tetrahedron 2015, 71, 3020–3026. [Google Scholar] [CrossRef]
- Thakker, A.M.; Sun, D. Biologically Plant-Based Pigments in Sustainable Innovations for Functional Textiles–The Role of Bioactive Plant Phytochemicals. J. Text. Sci. Fash. Technol. 2021, 8, 1–25. [Google Scholar] [CrossRef]
- Reis, A.C.C.; Silva, B.M.; de Moura, H.M.M.; Pereira, G.R.; Brandão, G.C. Anti-Zika virus activity and chemical characterization by ultra-high performance liquid chromatography (UPLC-DAD-UV-MS) of ethanol extracts in Tecoma species. BMC Complement. Med. Ther. 2020, 20, 246. [Google Scholar] [CrossRef]
- Da Silva, F.R.L.; Rodrigues, F.E.A.; Gomes, A.R.S.; Arriaga, A.M.C.; Mafezoli, J.; Lemos, T.L.G.; De Almeida, M.C.S.; Santiago, G.M.P.; Filho, R.B.; Da Costa, J.G.M.; et al. Phytochemical study, antioxidant and antibacterial activities of Stemodia maritima. Química Nova 2014, 37, 1474–1478. [Google Scholar] [CrossRef]
- Fan, W.; Qian, S.; Qian, P.; Li, X. Antiviral activity of luteolin against Japanese encephalitis virus. Virus Res. 2016, 220, 112–116. [Google Scholar] [CrossRef]
- Wang, S.; Ling, Y.; Yao, Y.; Zheng, G.; Chen, W. Luteolin inhibits respiratory syncytial virus replication by regulating the MiR-155/SOCS1/STAT1 signaling pathway. Virol. J. 2020, 17, 187. [Google Scholar] [CrossRef] [PubMed]
- Shawan, M.M.A.K.; Halder, S.K.; Hasan, A. Luteolin and abyssinone II as potential inhibitors of SARS-CoV-2: An in silico molecular modeling approach in battling the COVID-19 outbreak. Bull. Natl. Res. Cent. 2021, 45, 27. [Google Scholar] [CrossRef] [PubMed]
- Theerawatanasirikul, S.; Thangthamniyom, N.; Kuo, C.-J.; Semkum, P.; Phecharat, N.; Chankeeree, P.; Lekcharoensuk, P. Natural Phytochemicals, Luteolin and Isoginkgetin, Inhibit 3C Protease and Infection of FMDV, In Silico and In Vitro. Viruses 2021, 13, 2118. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Ham, S.; Kwon, T.H.; Kim, M.S.; Lee, D.H.; Kang, J.-W.; Oh, S.-R.; Yoon, D.-Y. Luteolin Induces Cell Cycle Arrest and Apoptosis Through Extrinsic and Intrinsic Signaling Pathways in MCF-7 Breast Cancer Cells. J. Environ. Pathol. Toxicol. Oncol. 2014, 33, 219–231. [Google Scholar] [CrossRef]
- Sato, Y.; Sasaki, N.; Saito, M.; Endo, N.; Kugawa, F.; Ueno, A. Luteolin Attenuates Doxorubicin-Induced Cytotoxicity to MCF-7 Human Breast Cancer Cells. Biol. Pharm. Bull. 2015, 38, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Magura, J.; Moodley, R.; Mackraj, I. The effect of hesperidin and luteolin isolated from Eriocephalus africanus on apoptosis, cell cycle and miRNA expression in MCF-7. J. Biomol. Struct. Dyn. 2020, 40, 1791–1800. [Google Scholar] [CrossRef]
- Rao, P.S.; Satelli, A.; Moridani, M.; Jenkins, M.; Rao, U.S. Luteolin induces apoptosis in multidrug resistant cancer cells without affecting the drug transporter function: Involvement of cell line-specific apoptotic mechanisms. Int. J. Cancer 2011, 130, 2703–2714. [Google Scholar] [CrossRef] [Green Version]
- Jeon, Y.-W.; Suh, Y.J. Synergistic apoptotic effect of celecoxib and luteolin on breast cancer cells. Oncol. Rep. 2012, 29, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-M.; Xie, K.-P.; Huo, H.-N.; Shang, F.; Zou, W.; Xie, M.-J. Luteolin inhibits proliferation induced by IGF-1 pathway dependent ER? in human breast cancer MCF-7 cells. Asian Pac. J. Cancer Prev. 2012, 13, 1431–1437. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S. Afr. J. Bot. 2020, 137, 257–264. [Google Scholar] [CrossRef]
- Romanová, D.; Vachálková, A.; Cipák, L.; Ovesná, Z.; Rauko, P. Study of antioxidant effect of apigenin, luteolin and quercetin by DNA protective method. Neoplasma 2001, 48, 104–107. [Google Scholar] [PubMed]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-Antioxidant Activity Relationships of Luteolin and Catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.H.; Hsieh, M.J.; Wu, C.-R.; Peng, W.-H.; Hsieh, M.-T.; Hsieh, C.-C. The synergistic effect of antioxidant interaction between luteolin and chlorogenic acid in Lonicera japonica. bioRxiv 2018, 418319. [Google Scholar]
- Wang, H.; Ding, Y.; Zhou, J.; Sun, X.; Wang, S. The in vitro and in vivo antiviral effects of salidroside from Rhodiola rosea L. against coxsackievirus B3. Phytomedicine 2009, 16, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Mishra, K.P.; Ganju, L. Salidroside exhibits anti-dengue virus activity by upregulating host innate immune factors. Arch. Virol. 2016, 161, 3331–3344. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Wu, Y.; Guo, H.; Zhang, Z.; He, Y. Salidroside Protects Against Influenza A Virus-Induced Acute Lung Injury in Mice. Dose-Response 2021, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Shi, A.; Fan, Z.; Du, Y. Salidroside inhibits the growth of human breast cancer in vitro and in vivo. Oncol. Rep. 2015, 33, 2553–2560. [Google Scholar] [CrossRef]
- Sun, A.-Q.; Ju, X.-L. Inhibitory effects of salidroside on MCF-7 breast cancer cells in vivo. J. Int. Med Res. 2020, 48, 1–12. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Qiu, S.; Yu, D.; Lin, S. Salidroside induces cell-cycle arrest and apoptosis in human breast cancer cells. Biochem. Biophys. Res. Commun. 2010, 398, 62–67. [Google Scholar] [CrossRef]
- Ju, L.; Wen, X.; Wang, C.; Wei, Y.; Peng, Y.; Ding, Y.; Feng, L.; Shu, L. Salidroside, A Natural Antioxidant, Improves β-Cell Survival and Function via Activating AMPK Pathway. Front. Pharmacol. 2017, 8, 749. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Tuo, Q.; Li, D.; Wang, X.; Li, X.; Zhang, Y.; Zhao, G.; Lin, F. Antioxidant Effects of Salidroside in the Cardiovascular System. Evidence-Based Complement. Altern. Med. 2020, 2020, 9568647. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wu, S.-J.; Liu, X.; Zhang, L.-L. Antioxidant effect of salidroside and its protective effect against furan-induced hepatocyte damage in mice. Food Funct. 2013, 4, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Jia, F.-Y.; Chen, X.; Wang, Z.-H.; Jin, W.-Y.; Yang, J. Salidroside alleviates oxidative stress and apoptosis via AMPK/Nrf2 pathway in DHT-induced human granulosa cell line KGN. Arch. Biochem. Biophys. 2021, 715, 109094. [Google Scholar] [CrossRef] [PubMed]
- Patel, F.; Spassieva, S.D. Side Effects in Cancer Therapy: Are Sphingolipids to Blame? Adv. Cancer Res. 2018, 140, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, R.; Vaz-Luis, I. Managing side effects in adjuvant endocrine therapy for breast cancer. Expert Rev. Anticancer Ther. 2018, 18, 1101–1112. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [Green Version]
- Das, G.; Patra, J.K.; Kang, S.-S.; Shin, H.-S. Pharmaceutical Importance of Some Promising Plant Species with Special Reference to the Isolation and Extraction of Bioactive Compounds: A Review. Curr. Pharm. Biotechnol. 2022, 23, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Pietrzak, W.; Nowak, R. Impact of Harvest Conditions and Host Tree Species on Chemical Composition and Antioxidant Activity of Extracts from Viscum album L. Molecules 2021, 26, 3741. [Google Scholar] [CrossRef]
- Do, Q.-D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talib, W.H.; Alsalahat, I.; Daoud, S.; Abutayeh, R.F.; Mahmod, A.I. Plant-Derived Natural Products in Cancer Research: Extraction, Mechanism of Action, and Drug Formulation. Molecules 2020, 25, 5319. [Google Scholar] [CrossRef] [PubMed]
- Abbes, Z.; El Abed, N.; Amri, M.; Kharrat, M.; Ben Hadj Ahmed, S. Antioxidant and antibacterial activities of the parasitic plants Orobanche foetida and Orobanche crenata collected on faba bean in Tunisia. J. Anim. Plant Sci. 2014, 24, 310–314. [Google Scholar]
- Abo-Qotb, S.M.S.; Hassanein, A.M.M.; Desoukey, S.Y.; Wanas, A.S.; Tawfik, H.M.; Orabi, M.A.A. In vivo anti-inflammatory and hepatoprotective activities of Orobanche crenata (Forssk.) aerial parts in relation to its phytomolecules. Nat. Prod. Res. 2020, 36, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Kokoska, L.; Havlik, J.; Valterova, I.; Sovova, H.; Sajfrtova, M.; Jankovska, I. Comparison of Chemical Composition and Antibacterial Activity of Nigella sativa Seed Essential Oils Obtained by Different Extraction Methods. J. Food Prot. 2008, 71, 2475–2480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Kott, A.F.; ElBealy, E.R.; Alshehri, A.S.; El-Kenawy, A.E.; Khalifa, H.S.; AlRamlawy, A.M. Salidroside induces cell apoptosis and inhibits the invasiveness of HT29 colorectal cells by regulating protein kinase R, NF-κB and STAT3. Cancer Biomark. 2021, 31, 13–25. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.-Z.; Lu, A.-X.; Zhang, K.-F.; Li, B.-J. Anticancer effect of salidroside on A549 lung cancer cells through inhibition of oxidative stress and phospho-p38 expression. Oncol. Lett. 2014, 7, 1159–1164. [Google Scholar] [CrossRef]
- Sun, K.X.; Xia, H.W.; Xia, R.L. Anticancer effect of salidroside on colon cancer through inhibiting JAK2/STAT3 signaling pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 615–621. [Google Scholar]
- Shang, H.; Wang, S.; Yao, J.; Guo, C.; Dong, J.; Liao, L. Salidroside inhibits migration and invasion of poorly differentiated thyroid cancer cells. Thorac. Cancer 2019, 10, 1469–1478. [Google Scholar] [CrossRef]
- Hu, X.; Lin, S.; Yu, D.; Qiu, S.; Zhang, X.; Mei, R. A preliminary study: The anti-proliferation effect of salidroside on different human cancer cell lines. Cell Biol. Toxicol. 2010, 26, 499–507. [Google Scholar] [CrossRef]
- Gest, C.; Joimel, U.; Huang, L.; Pritchard, L.-L.; Petit, A.; Dulong, C.; Buquet, C.; Hu, C.-Q.; Mirshahi, P.; Laurent, M.; et al. Rac3 induces a molecular pathway triggering breast cancer cell aggressiveness: Differences in MDA-MB-231 and MCF-7 breast cancer cell lines. BMC Cancer 2013, 13, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Ye, P.; Long, X. Differential Expression Profiles of the Transcriptome in Breast Cancer Cell Lines Revealed by Next Generation Sequencing. Cell. Physiol. Biochem. 2017, 44, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Naphong, C.; Pompimon, W.; Sombutsiri, P. Anticancer activity of isolated chemical constituents from Miliusa smithiae. Am. J. Appl. Sci. 2013, 10, 787–792. [Google Scholar] [CrossRef] [Green Version]
- Majumder, M.; Debnath, S.; Gajbhiye, R.L.; Saikia, R.; Gogoi, B.; Samanta, S.K.; Das, D.; Biswas, K.; Jaisankar, P.; Mukhopadhyay, R. Ricinus communis L. fruit extract inhibits migration/invasion, induces apoptosis in breast cancer cells and arrests tumor progression in vivo. Sci. Rep. 2019, 9, 14493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, M.J.; Chung, C.-K.; Jeong, Y.; Ham, S.-S. Anticancer activity of subfractions containing pure compounds of Chaga mushroom (Inonotus obliquus) extract in human cancer cells and in Balbc/c mice bearing Sarcoma-180 cells. Nutr. Res. Pr. 2010, 4, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Nogueira, V.; Hay, N. Molecular Pathways: Reactive Oxygen Species Homeostasis in Cancer Cells and Implications for Cancer Therapy. Clin. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, V.; Park, Y.; Chen, C.-C.; Xu, P.-Z.; Chen, M.-L.; Tonic, I.; Unterman, T.; Hay, N. Akt Determines Replicative Senescence and Oxidative or Oncogenic Premature Senescence and Sensitizes Cells to Oxidative Apoptosis. Cancer Cell 2008, 14, 458–470. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.-S.; Hong, M.-Z.; Ren, J.-L. Reactive oxygen species: A double-edged sword in oncogenesis. World J. Gastroenterol. 2009, 15, 1702–1707. [Google Scholar] [CrossRef]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Celeste Simon, M. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marengo, B.; Nitti, M.; Furfaro, A.L.; Colla, R.; De Ciucis, C.; Marinari, U.M.; Pronzato, M.A.; Traverso, N.; Domenicotti, C. Redox Homeostasis and Cellular Antioxidant Systems: Crucial Players in Cancer Growth and Therapy. Oxid. Med. Cell. Longev. 2016, 2016, 6235641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabała-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Iriti, M.; Wojtyczka, R.D.; Buszman, E.; Stojko, J. Flavonoids, bioactive components of propolis, exhibit cytotoxic activity and induce cell cycle arrest and apoptosis in human breast cancer cells MDA-MB-231 and MCF-7—A comparative study. Cell. Mol. Biol. 2018, 64, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shendge, A.; Chaudhuri, D.; Basu, T.; Mandal, N. A natural flavonoid, apigenin isolated from Clerodendrum viscosum leaves, induces G2/M phase cell cycle arrest and apoptosis in MCF-7 cells through the regulation of p53 and caspase-cascade pathway. Clin. Transl. Oncol. 2020, 23, 718–730. [Google Scholar] [CrossRef]
- Seo, H.-S.; Ku, J.M.; Choi, H.S.; Woo, J.-K.; Lee, B.H.; Kim, D.S.; Song, H.J.; Jang, B.-H.; Shin, Y.C.; Ko, S.-G. Apigenin overcomes drug resistance by blocking the signal transducer and activator of transcription 3 signaling in breast cancer cells. Oncol. Rep. 2017, 38, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Lecomte, S.; Demay, F.; Pham, T.H.; Moulis, S.; Efstathiou, T.; Chalmel, F.; Pakdel, F. Deciphering the Molecular Mechanisms Sustaining the Estrogenic Activity of the Two Major Dietary Compounds Zearalenone and Apigenin in ER-Positive Breast Cancer Cell Lines. Nutrients 2019, 11, 237. [Google Scholar] [CrossRef] [Green Version]
- Wen, L.; Zhao, Y.; Jiang, Y.; Yu, L.; Zeng, X.; Yang, J.; Tian, M.; Liu, H.; Yang, B. Identification of a flavonoid C-glycoside as potent antioxidant. Free Radic. Biol. Med. 2017, 110, 92–101. [Google Scholar] [CrossRef]
- Grigalius, I.; Petrikaite, V. Relationship between Antioxidant and Anticancer Activity of Trihydroxyflavones. Molecules 2017, 22, 2169. [Google Scholar] [CrossRef] [Green Version]
- Altamimi, M.A.; Hussain, A.; AlRajhi, M.; Alshehri, S.; Imam, S.S.; Qamar, W. Luteolin-Loaded Elastic Liposomes for Transdermal Delivery to Control Breast Cancer: In Vitro and Ex Vivo Evaluations. Pharmaceuticals 2021, 14, 1143. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, J.; Yang, F.; Wu, J.; Cai, R.; Wang, T.; Zhang, J. Effect of luteolin on the methylation status of the OPCML gene and cell growth in breast cancer cells. Exp. Ther. Med. 2018, 16, 3186–3194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, G.; Ge, R.; Li, Y.; Liu, S. Luteolin exhibits anti-breast cancer property through up-regulating miR-203. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3265–3271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Agócs, A.; Deli, J. Lutein Exerts Antioxidant and Anti-Inflammatory Effects and Influences Iron Utilization of BV-2 Microglia. Antioxidants 2021, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, H.-Q.; Guo, T.-T.; Li, Y.-H. Luteolin inhibits CVB3 replication through inhibiting inflammation. J. Asian Nat. Prod. Res. 2019, 22, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Khan, W.H.; Rathore, A.S. Synergistic Effects of Natural Compounds Toward Inhibition of SARS-CoV-2 3CL Protease. J. Chem. Inf. Model. 2021, 61, 5708–5718. [Google Scholar] [CrossRef]







| OCLE a (µg/mL) | Acyclovir (µg/mL) | ||
|---|---|---|---|
| CD50 b | Vero | 600 | OR c |
| HEp-2 | 600 | OR | |
| HCT-8 | 300 | OR | |
| ID50 d | Polio 1 | >600 | OR |
| Cox B1 | 100 | OR | |
| Cox B3 | 200 | OR | |
| ECHO 9 | >600 | OR | |
| RSV | >600 | OR | |
| Adeno 2 | >600 | OR | |
| Adeno 5 | >600 | OR | |
| HSV-1 | 50 | 0.12 | |
| HSV-2 | 100 | 0.36 | |
| OC-43 | >300 | OR |
| Chemical Name | Chemical Class | Chemical Structure | m/z (g/mol) | Polarity | Peak | RT (min) | Biological Activities |
|---|---|---|---|---|---|---|---|
| Acteoside | Phenylpropanoid glycosides |  | 624.205 | Pos | 625.21 | 23.23 | Antiviral [39,40] Antitumor (MCF-7) [41,42] Antioxidant [43] |
| Neg | 623.19 | ||||||
| Apigenin | Flavones |  | 270.053 | Pos | 271.06 | 35.97 | Antiviral [44] Antitumor (MCF-7) [45] Antioxidant [46] |
| Neg | 269.04 | ||||||
| Acutissimin A | Complex tannins |  | 1206.822 | Pos | 1207.14 | n.r. | Antiviral [47] Antioxidant [48] |
| Neg | 1205.10 | ||||||
| Crenatoside | Phenylpropanoid glycosides |  | 622.190 | Pos | n.r. | 54.02 | Antiviral [49] Antioxidant [50] |
| Neg | n.r. | ||||||
| Luteolin | Flavones |  | 286.048 | Pos | 287.05 | 21.85 | Antiviral [51,52,53,54] Antitumor (MCF-7) [55,56,57,58,59,60] Antioxidant [61,62,63,64] |
| Neg | 285.03 | ||||||
| Salidroside | O-glycosyl compounds |  | 300.121 | Pos | 301.12 | 35.10 | Antiviral [65,66,67] Antitumor (MCF-7) [68,69,70] Antioxidant [71,72,73,74] |
| Neg | 299.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genovese, C.; Garozzo, A.; D’Angeli, F.; Malfa, G.A.; Bellia, F.; Tomasello, B.; Nicolosi, D.; Malaguarnera, R.; Ronsisvalle, S.; Guadagni, F.; et al. Orobanche crenata Forssk. Extract Affects Human Breast Cancer Cell MCF-7 Survival and Viral Replication. Cells 2022, 11, 1696. https://doi.org/10.3390/cells11101696
Genovese C, Garozzo A, D’Angeli F, Malfa GA, Bellia F, Tomasello B, Nicolosi D, Malaguarnera R, Ronsisvalle S, Guadagni F, et al. Orobanche crenata Forssk. Extract Affects Human Breast Cancer Cell MCF-7 Survival and Viral Replication. Cells. 2022; 11(10):1696. https://doi.org/10.3390/cells11101696
Chicago/Turabian StyleGenovese, Carlo, Adriana Garozzo, Floriana D’Angeli, Giuseppe Antonio Malfa, Francesco Bellia, Barbara Tomasello, Daria Nicolosi, Roberta Malaguarnera, Simone Ronsisvalle, Fiorella Guadagni, and et al. 2022. "Orobanche crenata Forssk. Extract Affects Human Breast Cancer Cell MCF-7 Survival and Viral Replication" Cells 11, no. 10: 1696. https://doi.org/10.3390/cells11101696