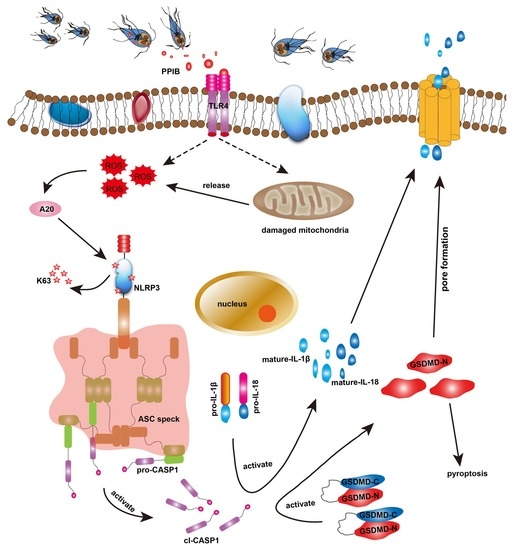
Giardia duodenalis and Its Secreted PPIB Trigger Inflammasome Activation and Pyroptosis in Macrophages through TLR4-Induced ROS Signaling and A20-Mediated NLRP3 Deubiquitination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Cell Culture
2.3. Parasite Culture
2.4. Lactate Dehydrogenase (LDH) Assay
2.5. Annexin V/Propidium Iodide (PI) Assay
2.6. Cell Viability Assays
2.7. Transmission Electron Microscopy (TEM) Detection
2.8. Reactive Oxygen Species (ROS) Detection
2.9. Detection of the Mitochondrial Membrane Potential (MMP)
2.10. Quantitative Real-Time PCR (qPCR) Analysis
2.11. Western Blot Analysis
2.12. Immunofluorescence Assays
2.13. Flow Cytometry for TLR Detection
2.14. Co-Immunoprecipitation (Co-IP) Analysis
2.15. RNA Interference
2.16. Prokaryotic Expression of Giardia-Secreted Proteins
2.17. Statistical Analysis
3. Results
3.1. Giardia-Induced Cell Death and Mitochondrial Damage in Macrophages
3.2. Giardia-Induced NLRP3/CASP1/GSDMD-Mediated Macrophage Pyroptosis
3.3. Giardia-Induced ROS/NLRP3/CASP1/GSDMD-Mediated Macrophage Pyroptosis
3.4. Involvement of A20-Mediated NLRP3 Deubiquitination in Giardia-Induced Macrophage Pyroptosis
3.5. Involvement of TLR4 Activation in Giardia-Induced Macrophage Pyroptosis
3.6. Giardia-Secreted PPIB Independently Induced Macrophage Pyroptosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fletcher, S.M.; Stark, D.; Harkness, J.; Ellis, J. Enteric protozoa in the developed world: A public health perspective. Clin. Microbiol. Rev. 2012, 25, 420–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minetti, C.; Chalmers, R.M.; Beeching, N.J.; Probert, C.; Lamden, K. Giardiasis. BMJ 2016, 355, i5369. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cama, V.A.; Mathison, B.A. Infections by intestinal Coccidia and Giardia duodenalis. Clin. Lab. Med. 2015, 35, 423–444. [Google Scholar] [CrossRef] [Green Version]
- Einarsson, E.; Ma’ayeh, S.; Svärd, S.G. An up-date on Giardia and giardiasis. Curr. Opin. Microbiol. 2016, 34, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.M.; Fink, M.Y.; Angelova, V.V. Recent insights into innate and adaptive immune responses to Giardia. Adv. Parasitol. 2019, 106, 171–208. [Google Scholar] [CrossRef]
- Zarebavani, M.; Dargahi, D.; Einollahi, N.; Dashti, N.; Mohebali, M.; Rezaeian, M. Serum levels of zinc, copper, vitamin B12, folate and immunoglobulins in individuals with giardiasis. Iran. J. Public Health 2012, 41, 47–53. [Google Scholar]
- Jiménez, J.C.; Fontaine, J.; Creusy, C.; Fleurisse, L.; Grzych, J.M.; Capron, M.; Dei-Cas, E. Antibody and cytokine responses to Giardia excretory/secretory proteins in Giardia intestinalis-infected BALB/c mice. Parasitol. Res. 2014, 113, 2709–2718. [Google Scholar] [CrossRef]
- Serradell, M.C.; Gargantini, P.R.; Saura, A.; Oms, S.R.; Rupil, L.L.; Berod, L.; Sparwasser, T.; Luján, H.D. Cytokines, antibodies, and histopathological profiles during Giardia infection and variant-specific surface protein-based vaccination. Infect. Immun. 2018, 86, e00773-17. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.Y.; Kim, J.; Noh, H.J.; Kim, H.P.; Park, S.J. Giardia lamblia binding immunoglobulin protein triggers maturation of dendritic cells via activation of TLR4-MyD88-p38 and ERK1/2 MAPKs. Parasite Immunol. 2014, 36, 627–646. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fang, R.; Wei, Z.; Wu, J.; Li, X.; Li, W. Giardia duodenalis induces apoptosis in intestinal epithelial cells via reactive oxygen species-mediated mitochondrial pathway in vitro. Pathogens 2020, 9, 693. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Z.; Fang, R.; Li, X.; Li, W. Giardia duodenalis induces extrinsic pathway of apoptosis in intestinal epithelial cells through activation of TNFR1 and K63 de-ubiquitination of RIP1 in vitro. Microb. Pathog. 2020, 149, 104315. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Gong, P.; Xia, F.; Li, L.; Yang, Z.; Li, J. TLR2-/- mice display decreased severity of giardiasis enhanced proinflammatory cytokines production dependent on AKT signal pathway. Front. Immunol. 2017, 8, 1186. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, X.; Li, G.; Gong, P.; Zhang, X.; Yang, Z.; Yang, J.; Li, J. Mouse macrophages capture and kill Giardia lamblia by means of releasing extracellular trap. Dev. Comp. Immunol. 2018, 88, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.Y.; Xie, K.X.; Wang, S.-L.; Yuan, L.W. Inflammatory caspase-related pyroptosis: Mechanism, regulation and therapeutic potential for inflammatory bowel disease. Gastroenterol. Rep. 2018, 6, 167–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Carvalho, R.V.H.; Zamboni, D.S. Inflammasome activation in response to intracellular protozoan parasites. Trends Parasitol. 2020, 36, 459–472. [Google Scholar] [CrossRef] [PubMed]
- He, W.T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.H.; Zhong, C.Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [Green Version]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Hayward, J.A.; Mathur, A.; Ngo, C.; Man, S.M. Cytosolic recognition of microbes and pathogens: Inflammasomes in action. Microbiol. Mol. Biol. Rev. 2018, 82, e00015-18. [Google Scholar] [CrossRef] [Green Version]
- Man, S.M.; Kanneganti, T.D. Regulation of inflammasome activation. Immunol. Rev. 2015, 265, 6–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijay, K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int. Immunopharmacol. 2018, 59, 391–412. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, G.; Yuan, Y.; Wu, G.; Wang, S.; Yuan, L. NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-κB signaling. Cell Death Dis. 2019, 10, 906. [Google Scholar] [CrossRef]
- Serradell, M.C.; Rupil, L.L.; Martino, R.A.; Prucca, C.G.; Carranza, P.G.; Saura, A.; Fernández, E.A.; Gargantini, P.R.; Tenaglia, A.H.; Petiti, J.P.; et al. Efficient oral vaccination by bioengineering virus-like particles with protozoan surface proteins. Nat. Commun. 2019, 10, 361. [Google Scholar] [CrossRef]
- Zhao, P.; Cao, L.; Wang, X.; Dong, J.; Zhang, N.; Li, X.; Li, J.; Zhang, X.; Gong, P. Extracellular vesicles secreted by Giardia duodenalis regulate host cell innate immunity via TLR2 and NLRP3 inflammasome signaling pathways. PLoS Negl. Trop. Dis. 2021, 15, e0009304. [Google Scholar] [CrossRef]
- Py, B.F.; Kim, M.S.; Vakifahmetoglu-Norberg, H.; Yuan, J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol. Cell 2013, 49, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, N.; Liu, Z.S.; Xue, W.; Bai, Z.F.; Wang, Q.Y.; Dai, J.; Liu, X.; Huang, Y.J.; Cai, H.; Zhan, X.Y.; et al. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol. Cell 2017, 68, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Ma’ayeh, S.; Peirasmaki, D.; Lundström-Stadelmann, B.; Hellman, L.; Svärd, S.G. Secreted Giardia intestinalis cysteine proteases disrupt intestinal epithelial cell junctional complexes and degrade chemokines. Virulence 2018, 9, 879–894. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Pierres, G.; Argüello-García, R.; Laredo-Cisneros, M.S.; Fonseca-Linán, R.; Gómez-Mondragón, M.; Inzunza-Arroyo, R.; Flores-Benítez, D.; Raya-Sandino, A.; Chavez-Munguía, B.; Ventura-Gallegos, J.L.; et al. Giardipain-1, a protease secreted by Giardia duodenalis trophozoites, causes junctional, barrier and apoptotic damage in epithelial cell monolayers. Int. J. Parasitol. 2018, 48, 621–639. [Google Scholar] [CrossRef]
- Faria, C.P.; Neves, B.M.; Lourenço, Á.; Cruz, M.T.; Martins, J.D.; Silva, A.; Pereira, S.; Sousa, M.d.C. Giardia lamblia decreases NF-κB p65 protein levels and modulates LPS-induced pro-Inflammatory response in macrophages. Sci. Rep. 2020, 10, 6234. [Google Scholar] [CrossRef] [Green Version]
- Dubourg, A.; Xia, D.; Winpenny, J.P.; Al Naimi, S.; Bouzid, M.; Sexton, D.W.; Wastling, J.M.; Hunter, P.R.; Tyler, K.M. Giardia secretome highlights secreted tenascins as a key component of pathogenesis. Gigascience 2018, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ma’ayeh, S.Y.; Liu, J.; Peirasmaki, D.; Hörnaeus, K.; Bergström Lind, S.; Grabherr, M.; Bergquist, J.; Svärd, S.G. Characterization of the Giardia intestinalis secretome during interaction with human intestinal epithelial cells: The impact on host cells. PLoS Negl. Trop. Dis. 2017, 11, e0006120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davids, B.J.; Gillin, F.D. Methods for Giardia culture, cryopreservation, encystation, and excystation in vitro. In Giardia: A Model Organism; Luján, H.D., Svärd, S., Eds.; Springer: Vienna, Austria, 2011; pp. 381–394. [Google Scholar]
- Volchuk, A.; Ye, A.; Chi, L.; Steinberg, B.E.; Goldenberg, N.M. Indirect regulation of HMGB1 release by gasdermin D. Nat. Commun. 2020, 11, 4561. [Google Scholar] [CrossRef]
- Dan Dunn, J.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, Y.; Zhang, P.; Li, Y.; Yang, Y.; Yang, Y.; Zhu, J.; Song, X.; Jiang, G.; Fan, J. Hemorrhagic shock primes for lung vascular endothelial cell pyroptosis: Role in pulmonary inflammation following LPS. Cell Death Dis. 2016, 7, e2363. [Google Scholar] [CrossRef]
- Miao, E.A.; Rajan, J.V.; Aderem, A. Caspase-1-induced pyroptotic cell death. Immunol. Rev. 2011, 243, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Eichholz, K.; Bru, T.; Tran, T.T.P.; Fernandes, P.; Welles, H.; Mennechet, F.J.D.; Manel, N.; Alves, P.; Perreau, M.; Kremer, E.J. Immune-complexed adenovirus induce AIM2-mediated pyroptosis in human dendritic cells. PLoS Pathog. 2016, 12, e1005871. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.; Sugimoto, N.; Tobe, T. Enteropathogenic Escherichia coli uses NleA to inhibit NLRP3 inflammasome activation. PLoS Pathog. 2015, 11, e1005121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef]
- Antao, A.M.; Tyagi, A.; Kim, K.S.; Ramakrishna, S. Advances in deubiquitinating enzyme inhibition and applications in cancer therapeutics. Cancers 2020, 12, 1579. [Google Scholar] [CrossRef]
- Zeng, X.; Gu, H.; Peng, L.; Yang, Y.; Wang, N.; Shi, Y.; Zou, Q. Transcriptome profiling of lung innate immune responses potentially associated with the pathogenesis of acute Lethal Pneumonia. Front. Immunol. 2020, 11, 708. [Google Scholar] [CrossRef] [Green Version]
- Man, S.M.; Karki, R.; Kanneganti, T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Romero, G.; Quintero, J.; Astiazarán-García, H.; Velazquez, C. Host defences against Giardia lamblia. Parasite Immunol. 2015, 37, 394–406. [Google Scholar] [CrossRef]
- Schiene-Fischer, C.; Aumüller, T.; Fischer, G. Peptide bond cis/trans isomerases: A biocatalysis perspective of conformational dynamics in proteins. Top. Curr. Chem. 2013, 328, 35–67. [Google Scholar] [CrossRef]
- Fanghänel, J.; Fischer, G. Insights into the catalytic mechanism of peptidyl prolyl cis/trans isomerases. Front. Biosci. 2004, 9, 3453–3478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Port, G.C.; Freitag, N.E. Identification of novel Listeria monocytogenes secreted virulence factors following mutational activation of the central virulence regulator, PrfA. Infect. Immun. 2007, 75, 5886–5897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ünal, C.M.; Steinert, M. FKBPs in bacterial infections. Biochim. Biophys. Acta 2015, 1850, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Basak, C.; Pathak, S.K.; Bhattacharyya, A.; Pathak, S.; Basu, J.; Kundu, M. The secreted peptidyl prolyl cistrans-isomerase HP0175 of Helicobacter pylori induces apoptosis of gastric epithelial cells in a TLR4- and apoptosis signal-regulating kinase 1-dependent manner. J. Immunol. 2005, 174, 5672–5680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amedei, A.; Munari, F.; Bella, C.D.; Niccolai, E.; Benagiano, M.; Bencini, L.; Cianchi, F.; Farsi, M.; Emmi, G.; Zanotti, G.; et al. Helicobacter pylori secreted peptidyl prolyl cis, trans-isomerase drives Th17 inflammation in gastric adenocarcinoma. Intern. Emerg. Med. 2014, 9, 303–309. [Google Scholar] [CrossRef]
- Gruber, E.J.; Leifer, C.A. Molecular regulation of TLR signaling in health and disease: Mechano-regulation of macrophages and TLR signaling. Innate Immun. 2020, 26, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Kanneganti, T.D. Inflammatory cell death in intestinal pathologies. Immunol. Rev. 2017, 280, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Cao, L.; Wang, X.; Li, J.; Dong, J.; Zhang, N.; Li, X.; Li, S.; Sun, M.; Zhang, X.; et al. Giardia duodenalis extracellular vesicles regulate the proinflammatory immune response in mouse macrophages in vitro via the MAPK, AKT and NF-κB pathways. Parasites Vectors 2021, 14, 358. [Google Scholar] [CrossRef] [PubMed]
- Gurung, P.; Li, B.; Subbarao Malireddi, R.K.; Lamkanfi, M.; Geiger, T.L.; Kanneganti, T.-D. Chronic TLR stimulation controls NLRP3 inflammasome activation through IL-10 mediated regulation of NLRP3 expression and Caspase-8 activation. Sci. Rep. 2015, 5, 14488. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.-I.; Kurnasov, O.; Natarajan, V.; Hong, M.; Gudkov, A.V.; Osterman, A.L.; Wilson, I.A. Structural basis of TLR5-flagellin recognition and signaling. Science 2012, 335, 859–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gong, P.; Zhang, X.; Li, S.; Lu, X.; Zhao, C.; Yu, Q.; Wei, Z.; Yang, Y.; Liu, Q.; et al. NLRP3 inflammasome participates in host response to infection. Front. Immunol. 2018, 9, 1791. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.Y.; Maloney, J.; Keselman, A.; Li, E.; Menegas, S.; Staniorski, C.; Singer, S.M. Proliferation of resident macrophages is dispensable for protection during infections. Immunohorizons 2019, 3, 412–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.; Luo, Z.Q. Post-translational regulation of ubiquitin signaling. J. Cell Biol. 2019, 218, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Kattah, M.G.; Malynn, B.A.; Ma, A. Ubiquitin-modifying enzymes and regulation of the inflammasome. J. Mol. Biol. 2017, 429, 3471–3485. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, S.; Kar, S.; Chande, A.G.; Mukhopadhyaya, R.; Das, P.K. Leishmania donovani exploits host deubiquitinating enzyme A20, a negative regulator of TLR signaling, to subvert host immune response. J. Immunol 2012, 189, 924–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatansever, F.; de Melo, W.C.M.A.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species-bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Q.; Liu, X.; Chen, C.; Zhang, X.; Xie, P.; Liu, Y.; Zhou, S.; Tang, J. Erlotinib protests against LPS-induced parthanatos through inhibiting macrophage surface TLR4 expression. Cell Death Discov. 2021, 7, 181. [Google Scholar] [CrossRef] [PubMed]
- Turchyn, L.R.; Baginski, T.J.; Renkiewicz, R.R.; Lesch, C.A.; Mobley, J.L. Phenotypic and functional analysis of murine resident and induced peritoneal macrophages. Comp. Med. 2007, 57, 574–580. [Google Scholar] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Yang, Y.; Fang, R.; Zhu, W.; Wu, J.; Li, X.; Patankar, J.V.; Li, W. Giardia duodenalis and Its Secreted PPIB Trigger Inflammasome Activation and Pyroptosis in Macrophages through TLR4-Induced ROS Signaling and A20-Mediated NLRP3 Deubiquitination. Cells 2021, 10, 3425. https://doi.org/10.3390/cells10123425
Liu L, Yang Y, Fang R, Zhu W, Wu J, Li X, Patankar JV, Li W. Giardia duodenalis and Its Secreted PPIB Trigger Inflammasome Activation and Pyroptosis in Macrophages through TLR4-Induced ROS Signaling and A20-Mediated NLRP3 Deubiquitination. Cells. 2021; 10(12):3425. https://doi.org/10.3390/cells10123425
Chicago/Turabian StyleLiu, Lin, Yongwu Yang, Rui Fang, Weining Zhu, Jingxue Wu, Xiaoyun Li, Jay V. Patankar, and Wei Li. 2021. "Giardia duodenalis and Its Secreted PPIB Trigger Inflammasome Activation and Pyroptosis in Macrophages through TLR4-Induced ROS Signaling and A20-Mediated NLRP3 Deubiquitination" Cells 10, no. 12: 3425. https://doi.org/10.3390/cells10123425