Plasma Biomarker Profiling in Heart Failure Patients with Preserved Ejection Fraction before and after Spironolactone Treatment: Results from the Aldo-DHF Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasma Biomarkers
2.2. Statistical Analysis
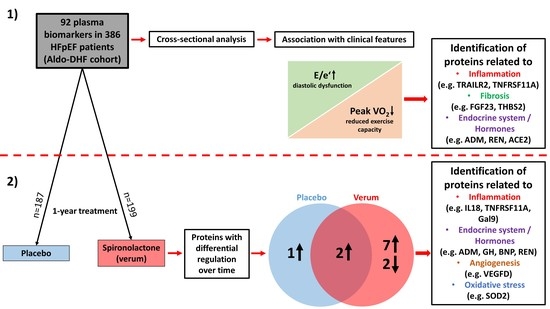
3. Results
3.1. Baseline
3.2. Effect of Spironolactone Treatment
3.3. Prediction of Beneficial Effects through Spironolactone Treatment
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A. The pathophysiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2014, 11, 507–515. [Google Scholar] [CrossRef]
- Senni, M.; Paulus, W.J.; Gavazzi, A.; Fraser, A.G.; Diez, J.; Solomon, S.D.; Smiseth, O.A.; Guazzi, M.; Lam, C.S.; Maggioni, A.P.; et al. New strategies for heart failure with preserved ejection fraction: The importance of targeted therapies for heart failure phenotypes. Eur. Heart J. 2014, 35, 2797–2815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverman, D.N.; Plante, T.B.; Infeld, M.; Callas, P.W.; Juraschek, S.P.; Dougherty, G.B.; Meyer, M. Association of beta-Blocker Use With Heart Failure Hospitalizations and Cardiovascular Disease Mortality Among Patients With Heart Failure With a Preserved Ejection Fraction: A Secondary Analysis of the TOPCAT Trial. JAMA Netw. Open 2019, 2, e1916598. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, F.; Wachter, R.; Schmidt, A.G.; Kraigher-Krainer, E.; Colantonio, C.; Kamke, W.; Duvinage, A.; Stahrenberg, R.; Durstewitz, K.; Loffler, M.; et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The Aldo-DHF randomized controlled trial. JAMA 2013, 309, 781–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacolley, P.; Safar, M.E.; Lucet, B.; Ledudal, K.; Labat, C.; Benetos, A. Prevention of aortic and cardiac fibrosis by spironolactone in old normotensive rats. J. Am. Coll. Cardiol. 2001, 37, 662–667. [Google Scholar] [CrossRef] [Green Version]
- Cezar, M.D.; Damatto, R.L.; Pagan, L.U.; Lima, A.R.; Martinez, P.F.; Bonomo, C.; Rosa, C.M.; Campos, D.H.; Cicogna, A.C.; Gomes, M.J.; et al. Early Spironolactone Treatment Attenuates Heart Failure Development by Improving Myocardial Function and Reducing Fibrosis in Spontaneously Hypertensive Rats. Cell. Physiol. Biochem. 2015, 36, 1453–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleland, J.G.F.; Ferreira, J.P.; Mariottoni, B.; Pellicori, P.; Cuthbert, J.; Verdonschot, J.A.J.; Petutschnigg, J.; Ahmed, F.Z.; Cosmi, F.; Brunner La Rocca, H.P.; et al. The effect of spironolactone on cardiovascular function and markers of fibrosis in people at increased risk of developing heart failure: The heart ‘OMics’ in AGEing (HOMAGE) randomized clinical trial. Eur. Heart J. 2021, 42, 684–696. [Google Scholar] [CrossRef] [PubMed]
- De Marco, C.; Claggett, B.L.; de Denus, S.; Zile, M.R.; Huynh, T.; Desai, A.S.; Sirois, M.G.; Solomon, S.D.; Pitt, B.; Rouleau, J.L.; et al. Impact of diabetes on serum biomarkers in heart failure with preserved ejection fraction: Insights from the TOPCAT trial. ESC Heart Fail. 2021, 8, 1130–1138. [Google Scholar] [CrossRef]
- Ravassa, S.; Trippel, T.; Bach, D.; Bachran, D.; Gonzalez, A.; Lopez, B.; Wachter, R.; Hasenfuss, G.; Delles, C.; Dominiczak, A.F.; et al. Biomarker-based phenotyping of myocardial fibrosis identifies patients with heart failure with preserved ejection fraction resistant to the beneficial effects of spironolactone: Results from the Aldo-DHF trial. Eur. J. Heart Fail. 2018, 20, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Tromp, J.; Westenbrink, B.D.; Ouwerkerk, W.; van Veldhuisen, D.J.; Samani, N.J.; Ponikowski, P.; Metra, M.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; et al. Identifying Pathophysiological Mechanisms in Heart Failure With Reduced Versus Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2018, 72, 1081–1090. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Verdonschot, J.; Wang, P.; Pizard, A.; Collier, T.; Ahmed, F.Z.; Brunner-La-Rocca, H.P.; Clark, A.L.; Cosmi, F.; Cuthbert, J.; et al. Proteomic and Mechanistic Analysis of Spironolactone in Patients at Risk for HF. JACC Heart Fail. 2021, 9, 268–277. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Ouwerkerk, W.; Santema, B.T.; van Veldhuisen, D.J.; Lang, C.C.; Ng, L.L.; Anker, S.D.; Dickstein, K.; Metra, M.; Cleland, J.G.F.; et al. Differences in biomarkers and molecular pathways according to age for patients with HFrEF. Cardiovasc. Res. 2020, 117, 2228–2236. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. ImerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Nishikimi, T.; Saito, Y.; Kitamura, K.; Ishimitsu, T.; Eto, T.; Kangawa, K.; Matsuo, H.; Omae, T.; Matsuoka, H. Increased plasma levels of adrenomedullin in patients with heart failure. J. Am. Coll. Cardiol. 1995, 26, 1424–1431. [Google Scholar] [CrossRef] [Green Version]
- Ter Maaten, J.M.; Kremer, D.; Demissei, B.G.; Struck, J.; Bergmann, A.; Anker, S.D.; Ng, L.L.; Dickstein, K.; Metra, M.; Samani, N.J.; et al. Bio-adrenomedullin as a marker of congestion in patients with new-onset and worsening heart failure. Eur. J. Heart Fail. 2019, 21, 732–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obokata, M.; Kane, G.C.; Reddy, Y.N.V.; Melenovsky, V.; Olson, T.P.; Jarolim, P.; Borlaug, B.A. The neurohormonal basis of pulmonary hypertension in heart failure with preserved ejection fraction. Eur. Heart J. 2019, 40, 3707–3717. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.C.; Sloan, A.; Isakova, T.; Gutierrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, H.; Li, X.; Li, Q.; Lin, H.; Chen, Z.; Xie, J.; Xuan, W.; Liao, W.; Bin, J.; Huang, X.; et al. FGF23 promotes myocardial fibrosis in mice through activation of beta-catenin. Oncotarget 2016, 7, 64649–64664. [Google Scholar] [CrossRef] [Green Version]
- Almahmoud, M.F.; Soliman, E.Z.; Bertoni, A.G.; Kestenbaum, B.; Katz, R.; Lima, J.A.C.; Ouyang, P.; Miller, P.E.; Michos, E.D.; Herrington, D.M. Fibroblast Growth Factor-23 and Heart Failure With Reduced Versus Preserved Ejection Fraction: MESA. J. Am. Heart Assoc. 2018, 7, e008334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edelmann, F.; Holzendorf, V.; Wachter, R.; Nolte, K.; Schmidt, A.G.; Kraigher-Krainer, E.; Duvinage, A.; Unkelbach, I.; Dungen, H.D.; Tschope, C.; et al. Galectin-3 in patients with heart failure with preserved ejection fraction: Results from the Aldo-DHF trial. Eur. J. Heart Fail. 2015, 17, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Tschope, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwanaga, Y.; Nishi, I.; Furuichi, S.; Noguchi, T.; Sase, K.; Kihara, Y.; Goto, Y.; Nonogi, H. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: Comparison between systolic and diastolic heart failure. J. Am. Coll. Cardiol. 2006, 47, 742–748. [Google Scholar] [CrossRef] [Green Version]
- Stenemo, M.; Nowak, C.; Byberg, L.; Sundstrom, J.; Giedraitis, V.; Lind, L.; Ingelsson, E.; Fall, T.; Arnlov, J. Circulating proteins as predictors of incident heart failure in the elderly. Eur. J. Heart Fail. 2018, 20, 55–62. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Savji, N.; Meijers, W.C.; Bartz, T.M.; Bhambhani, V.; Cushman, M.; Nayor, M.; Kizer, J.R.; Sarma, A.; Blaha, M.J.; Gansevoort, R.T.; et al. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC Heart Fail. 2018, 6, 701–709. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Brown, N.J. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat. Rev. Nephrol. 2013, 9, 459–469. [Google Scholar] [CrossRef]
- Guitart-Mampel, M.; Urquiza, P.; Borges, J.I.; Lymperopoulos, A.; Solesio, M.E. Impact of Aldosterone on the Failing Myocardium: Insights from Mitochondria and Adrenergic Receptors Signaling and Function. Cells 2021, 10, 1552. [Google Scholar] [CrossRef]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeffer, M.A.; Claggett, B.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; Gordeev, I.; et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015, 131, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Flint, K.M.; Shah, S.J.; Lewis, E.F.; Kao, D.P. Variation in clinical and patient-reported outcomes among complex heart failure with preserved ejection fraction phenotypes. ESC Heart Fail. 2020, 7, 811–824. [Google Scholar] [CrossRef] [Green Version]
- Belkin, M.N.; Blair, J.E.; Shah, S.J.; Alenghat, F.J. A composite metric for predicting benefit from spironolactone in heart failure with preserved ejection fraction. ESC Heart Fail. 2021, 8, 3495–3503. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Chiu, F.C.; Tsai, C.T.; Wang, Y.C.; Lai, L.P.; Hwang, J.J.; Lin, J.L. Poorer Exercise Accommodation of Regional Systolic Myocardial Motion after Spironolactone Treatment in Heart Failure Patients with Preserved Ejection Fraction and Ventricular Dyssynchrony. J. Clin. Med. 2021, 10, 3827. [Google Scholar] [CrossRef] [PubMed]
- Mares, A.; Rodriguez, T.; Deoker, A.; Lehker, A.; Mukherjee, D. Effect of Mineralocorticoid Receptor Antagonists in Heart Failure with Preserved Ejection Fraction and with Reduced Ejection Fraction—A Narrative Review. Curr. Vasc. Pharmacol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Elkholey, K.; Papadimitriou, L.; Butler, J.; Thadani, U.; Stavrakis, S. Effect of Obesity on Response to Spironolactone in Patients With Heart Failure With Preserved Ejection Fraction. Am. J. Cardiol. 2021, 146, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, T.; Kajio, H. Spironolactone Use and Improved Outcomes in Patients With Heart Failure With Preserved Ejection Fraction With Resistant Hypertension. J. Am. Heart Assoc. 2020, 9, e018827. [Google Scholar] [CrossRef]
- Suzuki, S.; Motoki, H.; Kanzaki, Y.; Maruyama, T.; Hashizume, N.; Kozuka, A.; Yahikozawa, K.; Kuwahara, K. Prognostic impact of mineralocorticoid receptor antagonist in patients with heart failure with preserved ejection fraction. ESC Heart Fail. 2020, 7, 2752–2761. [Google Scholar] [CrossRef]
- Kjeldsen, S.E.; von Lueder, T.G.; Smiseth, O.A.; Wachtell, K.; Mistry, N.; Westheim, A.S.; Hopper, I.; Julius, S.; Pitt, B.; Reid, C.M.; et al. Medical Therapies for Heart Failure With Preserved Ejection Fraction. Hypertension 2020, 75, 23–32. [Google Scholar] [CrossRef]
- Voors, A.A.; Kremer, D.; Geven, C.; Ter Maaten, J.M.; Struck, J.; Bergmann, A.; Pickkers, P.; Metra, M.; Mebazaa, A.; Dungen, H.D.; et al. Adrenomedullin in heart failure: Pathophysiology and therapeutic application. Eur. J. Heart Fail. 2019, 21, 163–171. [Google Scholar] [CrossRef]
- Napoli, R.; Guardasole, V.; Matarazzo, M.; Palmieri, E.A.; Oliviero, U.; Fazio, S.; Sacca, L. Growth hormone corrects vascular dysfunction in patients with chronic heart failure. J. Am. Coll. Cardiol. 2002, 39, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Niu, P.; Shindo, T.; Iwata, H.; Iimuro, S.; Takeda, N.; Zhang, Y.; Ebihara, A.; Suematsu, Y.; Kangawa, K.; Hirata, Y.; et al. Protective effects of endogenous adrenomedullin on cardiac hypertrophy, fibrosis, and renal damage. Circulation 2004, 109, 1789–1794. [Google Scholar] [CrossRef] [Green Version]
- Grimm, D.; Cameron, D.; Griese, D.P.; Riegger, G.A.; Kromer, E.P. Differential effects of growth hormone on cardiomyocyte and extracellular matrix protein remodeling following experimental myocardial infarction. Cardiovasc. Res. 1998, 40, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Zile, M.R.; Desantis, S.M.; Baicu, C.F.; Stroud, R.E.; Thompson, S.B.; McClure, C.D.; Mehurg, S.M.; Spinale, F.G. Plasma biomarkers that reflect determinants of matrix composition identify the presence of left ventricular hypertrophy and diastolic heart failure. Circ. Heart Fail. 2011, 4, 246–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsey, M.L.; Escobar, G.P.; Mukherjee, R.; Goshorn, D.K.; Sheats, N.J.; Bruce, J.A.; Mains, I.M.; Hendrick, J.K.; Hewett, K.W.; Gourdie, R.G.; et al. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation 2006, 113, 2919–2928. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Tian, Y.; Sun, L.; Zhou, L.; Xiao, L.; Tan, R.J.; Tian, J.; Fu, H.; Hou, F.F.; Liu, Y. Matrix Metalloproteinase-7 Is a Urinary Biomarker and Pathogenic Mediator of Kidney Fibrosis. J. Am. Soc. Nephrol. 2017, 28, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, R.; Yu, K.; Wang, Y.; Ding, Y.; Zhong, Y.; Zeng, Q. Galectin-9: A Suppressor of Atherosclerosis? Front. Immunol. 2020, 11, 604265. [Google Scholar] [CrossRef]
- Rickard, A.J.; Morgan, J.; Tesch, G.; Funder, J.W.; Fuller, P.J.; Young, M.J. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension 2009, 54, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Usher, M.G.; Duan, S.Z.; Ivaschenko, C.Y.; Frieler, R.A.; Berger, S.; Schutz, G.; Lumeng, C.N.; Mortensen, R.M. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J. Clin. Investig. 2010, 120, 3350–3364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraccarollo, D.; Thomas, S.; Scholz, C.J.; Hilfiker-Kleiner, D.; Galuppo, P.; Bauersachs, J. Macrophage Mineralocorticoid Receptor Is a Pleiotropic Modulator of Myocardial Infarct Healing. Hypertension 2019, 73, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Virdis, A.; Neves, M.F.; Amiri, F.; Viel, E.; Touyz, R.M.; Schiffrin, E.L. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension 2002, 40, 504–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, A.; Frangogiannis, N.G. Inflammatory Cytokines and Chemokines as Therapeutic Targets in Heart Failure. Cardiovasc. Drugs Ther. 2020, 34, 849–863. [Google Scholar] [CrossRef]
- Tamaki, S.; Mano, T.; Sakata, Y.; Ohtani, T.; Takeda, Y.; Kamimura, D.; Omori, Y.; Tsukamoto, Y.; Ikeya, Y.; Kawai, M.; et al. Interleukin-16 promotes cardiac fibrosis and myocardial stiffening in heart failure with preserved ejection fraction. PLoS ONE 2013, 8, e68893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, S.; Lee, P.J.; Mauro, A.G.; Mezzaroma, E.; Buzzetti, R.; Van Tassell, B.; Abbate, A.; Toldo, S. Interleukin-18 mediates cardiac dysfunction induced by western diet independent of obesity and hyperglycemia in the mouse. Nutr. Diabetes 2017, 7, e258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belperio, J.A.; Dy, M.; Murray, L.; Burdick, M.D.; Xue, Y.Y.; Strieter, R.M.; Keane, M.P. The role of the Th2 CC chemokine ligand CCL17 in pulmonary fibrosis. J. Immunol. 2004, 173, 4692–4698. [Google Scholar] [CrossRef] [Green Version]
- Glezeva, N.; Voon, V.; Watson, C.; Horgan, S.; McDonald, K.; Ledwidge, M.; Baugh, J. Exaggerated inflammation and monocytosis associate with diastolic dysfunction in heart failure with preserved ejection fraction: Evidence of M2 macrophage activation in disease pathogenesis. J. Card. Fail. 2015, 21, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Van Dyken, S.J.; Locksley, R.M. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: Roles in homeostasis and disease. Annu. Rev. Immunol. 2013, 31, 317–343. [Google Scholar] [CrossRef] [Green Version]



| Biomarker | E/e’ | Peak VO2 [mL/min/kg] | ||
|---|---|---|---|---|
| b-Coefficient (95%-CI) | p-Value | b-Coefficient (95%-CI) | p-Value | |
| Significant association with E/e’ and peak VO2 | ||||
| TRAILR2 | 1.55 (0.57 to 2.52) | 0.002 * | −1.35 (−2.18 to −0.52) | 0.001 * |
| FGF23 | 0.97 (0.33 to 1.61) | 0.003 * | −0.86 (−1.41 to −0.32) | 0.002 * |
| TNFRSF11A | 1.23 (0.37 to 2.09) | 0.005 * | −1.29 (−2.02 to −0.57) | 0.001 * |
| ADM | 0.90 (0.24 to 1.57) | 0.008 * | −1.52 (−2.08 to −0.96) | <0.001 * |
| HAOX1 | 0.56 (0.30 to 0.82) | <0.001 * | −0.29 (−0.51 to −0.07) | 0.011 |
| THBS2 | 3.31 (1.11 to 5.51) | 0.003 * | −2.39 (−4.26 to −0.52) | 0.012 |
| CA5A | 0.77 (0.35 to 1.19) | <0.001 * | −0.35 (−0.70 to −0.01) | 0.044 |
| ACE2 | 0.74 (0.14 to 1.33) | 0.015 | −0.71 (−1.21 to −0.21) | 0.006 |
| REN | 0.48 (0.10 to 0.85) | 0.013 | −0.35 (−0.67 to −0.04) | 0.029 |
| SPON2 | 2.73 (0.32 to 5.14) | 0.026 | −2.35 (−4.39 to −0.32) | 0.024 |
| Significant association with E/e’ only | ||||
| BNP | 1.12 (0.64 to 1.60) | <0.001 * | −0.11 (−0.47 to 0.25) | 0.542 |
| DECR1 | 0.64 (0.29 to 0.99) | <0.001 * | −0.00 (−0.30 to 0.30) | 0.996 |
| CTSL1 | 1.92 (0.85 to 2.98) | <0.001 * | 0.19 (−0.72 to 1.11) | 0.680 |
| KIM1 | 0.83 (0.36 to 1.30) | 0.001 * | −0.39 (−0.79 to 0.02) | 0.061 |
| IL27 | 2.15 (0.93 to 3.38) | 0.001 * | 0.20 (−0.86 to 1.25) | 0.715 |
| MARCO | 3.13 (1.33 to 4.92) | 0.001 * | −0.43 (−1.97 to 1.11) | 0.586 |
| FS | 1.12 (0.47 to 1.76) | 0.001 * | −0.14 (−0.69 to 0.42) | 0.630 |
| XCL1 | 1.11 (0.44 to 1.79) | 0.001 * | −0.44 (−1.02 to −0.14) | 0.137 |
| SORT1 | 2.34 (0.91 to 3.78) | 0.001 * | −0.28 (−1.51 to 0.95) | 0.658 |
| FABP2 | 0.81 (0.31 to 1.30) | 0.001 * | 0.33 (−0.09 to 0.75) | 0.126 |
| IL18 | 1.01 (0.32 to 1.69) | 0.004 * | 0.01 (−0.58 to 0.60) | 0.975 |
| MERTK | 1.50 (0.48 to 2.53) | 0.004 * | 0.04 (−0.83 to 0.92) | 0.924 |
| GT | 0.91 (0.28 to 1.54) | 0.005 * | 0.32 (−0.22 to 0.86) | 0.245 |
| CCL3 | 0.84 (0.23 to 1.44) | 0.007 * | −0.45 (−0.97 to 0.06) | 0.083 |
| HO1 | 1.15 (0.30 to 2.00) | 0.008 * | 0.13 (−0.59 to 0.86) | 0.721 |
| CD4 | 1.74 (0.42 to 3.05) | 0.010 * | −0.92 (−2.04 to 0.20) | 0.106 |
| MMP7 | 1.73 (0.32 to 3.14) | 0.017 | −1.08 (−2.28 to 0.12) | 0.078 |
| DCN | 2.09 (0.35 to 3.83) | 0.019 | 0.73 (−0.75 to 2.22) | 0.331 |
| PRELP | 2.30 (0.32 to 4.29) | 0.023 | −1.11 (−2.80 to 0.58) | 0.197 |
| SLAMF7 | 0.66 (0.08 to 1.24) | 0.027 | 0.09 (−0.40 to 0.59) | 0.714 |
| AGRP | 1.23 (0.13 to 2.33) | 0.029 | 0.15 (−0.79 to 1.08) | 0.761 |
| TNFRSF13B | 1.12 (0.10 to 2.15) | 0.032 | −0.46 (−1.33 to 0.42) | 0.305 |
| RAGE | 1.01 (0.04 to 1.97) | 0.041 | −0.24 (−1.06 to 0.58) | 0.570 |
| Significant association with peak VO2 only | ||||
| LEP | 0.47 (−0.01 to 0.94) | 0.054 | −1.44 (−1.81 to −1.06) | <0.001 * |
| IL1ra | 0.33 (−0.22 to 0.87) | 0.240 | −1.17 (−1.62 to −0.73) | <0.001 * |
| Gal9 | 1.13 (−0.15 to 2.40) | 0.082 | −2.25 (−3.31 to −1.19) | <0.001 * |
| FGF21 | 0.19 (−0.09 to 0.47) | 0.181 | −0.41 (−0.64 to −0.18) | 0.001 * |
| IL6 | 0.43 (−0.04 to 0.89) | 0.070 | −0.54 (−0.93 to −0.15) | 0.007 |
| PSGL1 | 1.17 (−0.27 to 2.60) | 0.111 | −1.57 (−2.77 to −0.36) | 0.011 |
| PDGF subunit B | 0.32 (−0.08 to 0.72) | 0.113 | 0.40 (0.06 to 0.73) | 0.021 |
| CXCL1 | 0.16 (−0.37 to 0.69) | 0.561 | 0.50 (0.05 to 0.95) | 0.028 |
| VSIG2 | 0.22 (−0.41 to 0.84) | 0.490 | −0.55 (−1.08 to −0.03) | 0.039 |
| ANGPT1 | 0.10 (−0.38 to 0.58) | 0.682 | 0.43 (0.02 to 0.83) | 0.041 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnelle, M.; Leha, A.; Eidizadeh, A.; Fuhlrott, K.; Trippel, T.D.; Hashemi, D.; Toischer, K.; Wachter, R.; Herrmann-Lingen, C.; Hasenfuß, G.; et al. Plasma Biomarker Profiling in Heart Failure Patients with Preserved Ejection Fraction before and after Spironolactone Treatment: Results from the Aldo-DHF Trial. Cells 2021, 10, 2796. https://doi.org/10.3390/cells10102796
Schnelle M, Leha A, Eidizadeh A, Fuhlrott K, Trippel TD, Hashemi D, Toischer K, Wachter R, Herrmann-Lingen C, Hasenfuß G, et al. Plasma Biomarker Profiling in Heart Failure Patients with Preserved Ejection Fraction before and after Spironolactone Treatment: Results from the Aldo-DHF Trial. Cells. 2021; 10(10):2796. https://doi.org/10.3390/cells10102796
Chicago/Turabian StyleSchnelle, Moritz, Andreas Leha, Abass Eidizadeh, Katharina Fuhlrott, Tobias D. Trippel, Djawid Hashemi, Karl Toischer, Rolf Wachter, Christoph Herrmann-Lingen, Gerd Hasenfuß, and et al. 2021. "Plasma Biomarker Profiling in Heart Failure Patients with Preserved Ejection Fraction before and after Spironolactone Treatment: Results from the Aldo-DHF Trial" Cells 10, no. 10: 2796. https://doi.org/10.3390/cells10102796