Insights into Cadmium-Induced Morphophysiological Disorders in Althea rosea Cavan and Its Phytoremediation through the Exogeneous Citric Acid
Abstract
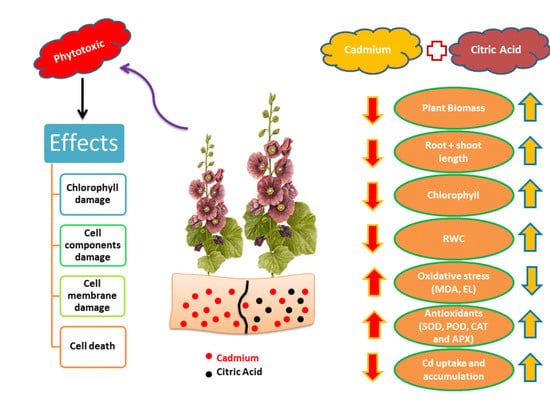
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Evaluation of Agronomic Characteristics
2.3. Determination of Chlorophyll and Carotenoid Contents
2.4. Leaf Relative Water Content (RWC)
2.5. Proline Content Estimation
2.6. Determination of Antioxidants Enzymes
2.7. Determination of MDA and Relative Electrolyte Leakage
2.8. Determination of Cd Concentration
2.9. Statistical Analysis
3. Results
3.1. Variation of Growth
3.2. Chlorophyll and Carotenoids
3.3. MDA and REL Contents
3.4. Antioxidants Enzyme
3.5. Proline Content
3.6. Plant Biomass and Distribution of Cd
3.7. Pearson’s Correlation
3.8. Principal Component Analysis
4. Discussion
4.1. Plant Biomass
4.2. Cd Accumulation
4.3. Chlorophyll and Carotenoids
4.4. Oxidative Stress Due to Cd and ROS Production
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ehsan, S.; Ali, S.; Noureen, S.; Mahmood, K.; Farid, M.; Ishaque, W.; Shakoor, M.B.; Rizwan, M. Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol. Environ. Saf. 2014, 106, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Artiola, J.; Walworth, J.; Musil, S.; Crimmins, M. Soil and land pollution. In Environmental and Pollution Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 219–235. [Google Scholar]
- Zainab, N.; Khan, A.A.; Azeem, M.A.; Ali, B.; Wang, T.; Shi, F.; Alghanem, S.M.; Hussain Munis, M.F.; Hashem, M.; Alamri, S. PGPR-mediated plant growth attributes and metal extraction ability of Sesbania sesban L. in industrially contaminated soils. Agronomy 2021, 11, 1820. [Google Scholar] [CrossRef]
- Cheng, S. Heavy metal pollution in China: Origin, pattern and control. Environ. Sci. Pollut. Res. 2003, 10, 192–198. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ae, N.; Freitas, H. Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere 2009, 77, 153–160. [Google Scholar] [CrossRef]
- Aioub, A.A.; Li, Y.; Qie, X.; Zhang, X.; Hu, Z. Reduction of soil contamination by cypermethrin residues using phytoremediation with Plantago major and some surfactants. Environ. Sci. Eur. 2019, 31, 26. [Google Scholar] [CrossRef] [Green Version]
- Diels, L.; Van der Lelie, N.; Bastiaens, L. New developments in treatment of heavy metal contaminated soils. Rev. Environ. Sci. Biotechnol. 2002, 1, 75–82. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Ali, S.; Farid, M.; Farooq, M.A.; Tauqeer, H.M.; Iftikhar, U.; Hannan, F.; Bharwana, S.A. Heavy metal pollution, a global problem and its remediation by chemically enhanced phytoremediation: A review. J. Biodivers. Environ. Sci. 2013, 3, 12–20. [Google Scholar]
- Mahdavian, K.; Ghaderian, S.M.; Schat, H. Pb accumulation, Pb tolerance, antioxidants, thiols, and organic acids in metallicolous and non-metallicolous Peganum harmala L. under Pb exposure. Environ. Exp. Bot. 2016, 126, 21–31. [Google Scholar] [CrossRef]
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Tolerance and hyperaccumulation of cadmium by a wild, unpalatable herb Coronopus didymus (L.) Sm.(Brassicaceae). Ecotoxicol. Environ. Saf. 2017, 135, 209–215. [Google Scholar] [CrossRef]
- Farid, M.; Shakoor, M.B.; Ehsan, S.; Ali, S.; Zubair, M.; Hanif, M. Morphological, physiological and biochemical responses of different plant species to Cd stress. Int. J. Chem. Biochem. Sci. 2013, 3, 53–60. [Google Scholar]
- Gill, S.S.; Khan, N.A.; Tuteja, N. Differential cadmium stress tolerance in five Indian mustard (Brassica juncea L.) cultivars: An evaluation of the role of antioxidant machinery. Plant Signal. Behav. 2011, 6, 293–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atafar, Z.; Mesdaghinia, A.; Nouri, J.; Homaee, M.; Yunesian, M.; Ahmadimoghaddam, M.; Mahvi, A.H. Effect of fertilizer application on soil heavy metal concentration. Environ. Monit. Assess. 2010, 160, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Ghosh, S.; Malinska, H.; Zivcak, M.; Brestic, M. Physiological and molecular mechanisms of metal accumulation in hyperaccumulator plants. Physiol. Plant. 2021, 173, 148–166. [Google Scholar] [CrossRef] [PubMed]
- Skuza, L.; Szućko-Kociuba, I.; Filip, E.; Bożek, I. Natural Molecular Mechanisms of Plant Hyperaccumulation and Hypertolerance towards Heavy Metals. Int. J. Mol. Sci. 2022, 23, 9335. [Google Scholar] [CrossRef]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 35. [Google Scholar] [CrossRef] [Green Version]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Hunter, P. Essentially deadly: Living with toxic elements: Humans and plants have evolved various mechanisms to deal with and even adopt toxic heavy metals. EMBO Rep. 2015, 16, 1605–1608. [Google Scholar] [CrossRef] [Green Version]
- Palmer, C.M.; Guerinot, M.L. Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat. Chem. Biol. 2009, 5, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Thomine, S.; Vert, G. Iron transport in plants: Better be safe than sorry. Curr. Opin. Plant Biol. 2013, 16, 322–327. [Google Scholar] [CrossRef]
- Niesche, R.; Haase, M. Emotions and ethics: A Foucauldian framework for becoming an ethical educator. Educ. Philos. Theory 2012, 44, 276–288. [Google Scholar] [CrossRef]
- Yaashikaa, P.; Kumar, P.S.; Jeevanantham, S.; Saravanan, R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ. Pollut. 2022, 301, 119035. [Google Scholar] [CrossRef] [PubMed]
- Raskin, I.; Kumar, P.N.; Dushenkov, S.; Salt, D.E. Bioconcentration of heavy metals by plants. Curr. Opin. Biotechnol. 1994, 5, 285–290. [Google Scholar] [CrossRef]
- Krouma, A.; Drevon, J.-J.; Abdelly, C. Genotypic variation of N2-fixing common bean (Phaseolus vulgaris L.) in response to iron deficiency. J. Plant Physiol. 2006, 163, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Mohammad, F.; Shafi, M.; Bakht, J.; Zhou, W. Effects of cadmium and salinity on growth and photosynthesis parameters of Brassica species. Pak. J. Bot. 2011, 43, 333–340. [Google Scholar]
- Mehmood, S.; Khan, A.A.; Shi, F.; Tahir, M.; Sultan, T.; Munis, M.F.H.; Kaushik, P.; Alyemeni, M.N.; Chaudhary, H.J. Alleviation of salt stress in wheat seedlings via multifunctional Bacillus aryabhattai PM34: An in-vitro study. Sustainability 2021, 13, 8030. [Google Scholar] [CrossRef]
- Aioub, A.A.; Zuo, Y.; Aioub, A.A.; Hu, Z. Biochemical and phytoremediation of Plantago major L. to protect tomato plants from the contamination of cypermethrin pesticide. Environ. Sci. Pollut. Res. 2021, 28, 43992–44001. [Google Scholar] [CrossRef]
- Zainab, N.; Din, B.U.; Javed, M.T.; Afridi, M.S.; Mukhtar, T.; Kamran, M.A.; Khan, A.A.; Ali, J.; Jatoi, W.N.; Munis, M.F.H. Deciphering metal toxicity responses of flax (Linum usitatissimum L.) with exopolysaccharide and ACC-deaminase producing bacteria in industrially contaminated soils. Plant Physiol. Biochem. 2020, 152, 90–99. [Google Scholar] [CrossRef]
- Zeng, F.; Mallhi, Z.I.; Khan, N.; Rizwan, M.; Ali, S.; Ahmad, A.; Hussain, A.; Alsahli, A.A.; Alyemeni, M.N. Combined Citric Acid and Glutathione Augments Lead (Pb) Stress Tolerance and Phytoremediation of Castorbean through Antioxidant Machinery and Pb Uptake. Sustainability 2021, 13, 4073. [Google Scholar] [CrossRef]
- Ali, B.; Gill, R.A.; Yang, S.; Gill, M.B.; Farooq, M.A.; Liu, D.; Daud, M.K.; Ali, S.; Zhou, W. Regulation of cadmium-induced proteomic and metabolic changes by 5-aminolevulinic acid in leaves of Brassica napus L. PLoS ONE 2015, 10, e0123328. [Google Scholar] [CrossRef]
- Bareen, F. Chelate assisted phytoextraction using oilseed brassicas. Environ. Pollut. 2012, 21, 289–311. [Google Scholar]
- Hayat, K.; Menhas, S.; Bundschuh, J.; Zhou, P.; Niazi, N.K.; Amna; Hussain, A.; Hayat, S.; Ali, H.; Wang, J. Plant growth promotion and enhanced uptake of Cd by combinatorial application of Bacillus pumilus and EDTA on Zea mays L. Int. J. Phytoremediat. 2020, 22, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- de Melo, É.E.C.; do Nascimento, C.W.A.; de Aguiar Accioly, A.M.; Santos, A.C.Q. Phytoextraction and fractionation of heavy metals in soil after multiple applications of natural chelants. Sci. Agric. 2008, 65, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Wuana, R.; Okieimen, F.; Imborvungu, J. Removal of heavy metals from a contaminated soil using organic chelating acids. Int. J. Environ. Sci. Technol. 2010, 7, 485–496. [Google Scholar] [CrossRef] [Green Version]
- Freitas, E.V.; Nascimento, C.W.; Souza, A.; Silva, F.B. Citric acid-assisted phytoextraction of lead: A field experiment. Chemosphere 2013, 92, 213–217. [Google Scholar] [CrossRef]
- Turgut, C.; Pepe, M.K.; Cutright, T.J. The effect of EDTA and citric acid on phytoremediation of Cd, Cr, and Ni from soil using Helianthus annuus. Environ. Pollut. 2004, 131, 147–154. [Google Scholar] [CrossRef]
- Najeeb, U.; Jilani, G.; Ali, S.; Sarwar, M.; Xu, L.; Zhou, W. Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J. Hazard. Mater. 2011, 186, 565–574. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Q.; Wang, X.; Zhang, Q.; Sun, T. Potential analysis of ornamental plant resources applied to contaminated soil remediation. In Floriculture, Ornamental and Plant Biotechnology; Global Science Books, Ltd.: Middlesec, UK, 2006; pp. 245–252. [Google Scholar]
- Ma, Y. The role of domestic floriculture in prevention and treatment of pollution. J. Changchun Univ. 2003, 13, 21–29. [Google Scholar]
- Liu, J.-N.; Zhou, Q.-X.; Sun, T.; Ma, L.Q.; Wang, S. Identification and chemical enhancement of two ornamental plants for phytoremediation. Bull. Environ. Contam. Toxicol. 2008, 80, 260–265. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Cao, X.; Xiao, X.; Liu, Y.; Shi, L. Phytostabilization potential of ornamental plants grown in soil contaminated with cadmium. Int. J. Phytoremediat. 2018, 20, 311–320. [Google Scholar] [CrossRef]
- Khan, A.A.; Wang, T.; Hussain, T.; Ali, F.; Shi, F.; Latef, A.A.H.A.; Ali, O.M.; Hayat, K.; Mehmood, S.; Zainab, N. Halotolerant-Koccuria rhizophila (14asp)-induced amendment of salt stress in pea plants by limiting Na+ uptake and elevating production of antioxidants. Agronomy 2021, 11, 1907. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Balestri, M.; Bottega, S.; Spanò, C. Response of Pteris vittata to different cadmium treatments. Acta Physiol. Plant. 2014, 36, 767–775. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Aebi, H. Formation or Removal of Oxygen Radicals. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Zhang, H.; Jiang, Y.; He, Z.; Ma, M. Cadmium accumulation and oxidative burst in garlic (Allium sativum). J. Plant Physiol. 2005, 162, 977–984. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K.; Arora, K. Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul. 2007, 53, 65–73. [Google Scholar] [CrossRef]
- Cheraghi, M.; Lorestani, B.; Khorasani, N.; Yousefi, N.; Karami, M. Findings on the phytoextraction and phytostabilization of soils contaminated with heavy metals. Biol. Trace Elem. Res. 2011, 144, 1133–1141. [Google Scholar] [CrossRef]
- Zubair, M.; Ramzani, P.M.A.; Rasool, B.; Khan, M.A.; Akhtar, I.; Turan, V.; Tauqeer, H.M.; Farhad, M.; Khan, S.A.; Iqbal, J. Efficacy of chitosan-coated textile waste biochar applied to Cd-polluted soil for reducing Cd mobility in soil and its distribution in moringa (Moringa oleifera L.). J. Environ. Manag. 2021, 284, 112047. [Google Scholar] [CrossRef]
- Chen, L.; Long, C.; Wang, D.; Yang, J. Phytoremediation of cadmium (Cd) and uranium (U) contaminated soils by Brassica juncea L. enhanced with exogenous application of plant growth regulators. Chemosphere 2020, 242, 125112. [Google Scholar] [CrossRef] [PubMed]
- Al Mahmud, J.; Hasanuzzaman, M.; Nahar, K.; Bhuyan, M.B.; Fujita, M. Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: Coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol. Environ. Saf. 2018, 147, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Andrades-Moreno, L. Accumulation and tolerance characteristics of cadmium in a halophytic Cd-hyperaccumulator, Arthrocnemum macrostachyum. J. Hazard. Mater. 2010, 184, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Tusei, C. The effects of citric acid on pH and nutrient uptake in wheatgrass (Triticum aestivum). IdeaFest Interdiscip. J. Creat. Work. Res. Humboldt State Univ. 2019, 3, 7. [Google Scholar]
- Yadav, S. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Ehlert, C.; Maurel, C.; Tardieu, F.; Simonneau, T. Aquaporin-mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration. Plant Physiol. 2009, 150, 1093–1104. [Google Scholar] [CrossRef] [Green Version]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A comprehensive review on the heavy metal toxicity and sequestration in plants. Biomolecules 2021, 12, 43. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Z.; Seleiman, M.F.; Rizwan, M.; YavaŞ, İ.; Alhammad, B.A.; Shami, A.; Hasanuzzaman, M.; Kalderis, D. Glycine betaine accumulation, significance and interests for heavy metal tolerance in plants. Plants 2020, 9, 896. [Google Scholar] [CrossRef]
- Gupta, D.; Nicoloso, F.; Schetinger, M.; Rossato, L.; Pereira, L.; Castro, G.; Srivastava, S.; Tripathi, R. Antioxidant defense mechanism in hydroponically grown Zea mays seedlings under moderate lead stress. J. Hazard. Mater. 2009, 172, 479–484. [Google Scholar] [CrossRef]
- Muhammad, D.; Chen, F.; Zhao, J.; Zhang, G.; Wu, F. Comparison of EDTA-and citric acid-enhanced phytoextraction of heavy metals in artificially metal contaminated soil by Typha angustifolia. Int. J. Phytoremediat. 2009, 11, 558–574. [Google Scholar] [CrossRef]
- Gao, Y.; Miao, C.; Xia, J.; Luo, C.; Mao, L.; Zhou, P.; Shi, W. Effect of citric acid on phytoextraction and antioxidative defense in Solanum nigrum L. as a hyperaccumulator under Cd and Pb combined pollution. Environ. Earth Sci. 2012, 65, 1923–1932. [Google Scholar] [CrossRef]
- Sabir, M.; Hanafi, M.M.; Zia-Ur-Rehman, M.; Saifullah; Ahmad, H.R.; Hakeem, K.R.; Aziz, T. Comparison of low-molecular-weight organic acids and ethylenediaminetetraacetic acid to enhance phytoextraction of heavy metals by maize. Commun. Soil Sci. Plant Anal. 2014, 45, 42–52. [Google Scholar] [CrossRef]
- Najeeb, U.; Xu, L.; Ali, S.; Jilani, G.; Gong, H.; Shen, W.; Zhou, W. Citric acid enhances the phytoextraction of manganese and plant growth by alleviating the ultrastructural damages in Juncus effusus L. J. Hazard. Mater. 2009, 170, 1156–1163. [Google Scholar] [CrossRef]
- Pena, L.B.; Pasquini, L.A.; Tomaro, M.L.; Gallego, S.M. Proteolytic system in sunflower (Helianthus annuus L.) leaves under cadmium stress. Plant Sci. 2006, 171, 531–537. [Google Scholar] [CrossRef]
- Aghaz, M.; Bandehagh, A.; Aghazade, E.; Toorchi, M.; Ghassemi-Gholezani, K. Effects of cadmium stress on some growth and physiological characteristics in dill (Anethum graveolens) ecotypes. Int. J. Agric. 2013, 3, 409. [Google Scholar]
- Lu, L.-L.; Tian, S.-K.; Yang, X.-E.; Peng, H.-Y.; Li, T.-Q. Improved cadmium uptake and accumulation in the hyperaccumulator Sedum alfredii: The impact of citric acid and tartaric acid. J. Zhejiang Univ. Sci. B 2013, 14, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Shan, X.-Q.; Zhang, J.; Xie, Y.-N.; Pei, Z.-G.; Zhang, S.-Z.; Zhu, Y.-G.; Wen, B. Organic acids promote the uptake of lanthanum by barley roots. New Phytol. 2005, 165, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Din, M.; Ullah, A.; Sabagh, A.E.; Hossain, A.; Llanes, A.; Liu, L. Copper-induced oxidative stress, initiation of antioxidants and phytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China. Environ. Sci. Pollut. Res. 2020, 27, 5211–5221. [Google Scholar] [CrossRef] [PubMed]
- Babushkina, E.A.; Belokopytova, L.V.; Grachev, A.M.; Meko, D.M.; Vaganov, E.A. Variation of the hydrological regime of Bele-Shira closed basin in Southern Siberia and its reflection in the radial growth of Larix sibirica. Reg. Environ. Chang. 2017, 17, 1725–1737. [Google Scholar] [CrossRef]
- Parmar, P.; Kumari, N.; Sharma, V. Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot. Stud. 2013, 54, 45. [Google Scholar] [CrossRef] [Green Version]
- Kanwal, U.; Ali, S.; Shakoor, M.B.; Farid, M.; Hussain, S.; Yasmeen, T.; Adrees, M.; Bharwana, S.A.; Abbas, F. EDTA ameliorates phytoextraction of lead and plant growth by reducing morphological and biochemical injuries in Brassica napus L. under lead stress. Environ. Sci. Pollut. Res. 2014, 21, 9899–9910. [Google Scholar] [CrossRef] [PubMed]
- Mahdavian, K. Effect of citric acid on antioxidant activity of red bean (Phaseolus calcaratus L.) under Cr+6 stress. S. Afr. J. Bot. 2021, 139, 83–91. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Saeed, R.; Rizwan, M.; Bukhari, S.A.H.; Abbasi, G.H.; Hussain, A.; Ali, B.; Zamir, M.S.I.; Ahmad, I. Combined application of citric acid and 5-aminolevulinic acid improved biomass, photosynthesis and gas exchange attributes of sunflower (Helianthus annuus L.) grown on chromium contaminated soil. Int. J. Phytoremediat. 2019, 21, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Hua, S.; Shamsi, I.H.; Jilani, G.; Li, Y.; Jiang, L. Cadmium-induced stress on the seed germination and seedling growth of Brassica napus L., and its alleviation through exogenous plant growth regulators. Plant Growth Regul. 2009, 58, 47–59. [Google Scholar] [CrossRef]
- Haouari, C.C.; Nasraoui, A.H.; Bouthour, D.; Houda, M.D.; Daieb, C.B.; Mnai, J.; Gouia, H. Response of tomato (Solanum lycopersicon) to cadmium toxicity: Growth, element uptake, chlorophyll content and photosynthesis rate. Afr. J. Plant Sci. 2012, 6, 1–7. [Google Scholar]
- Kurtyka, R.; Burdach, Z.; Siemieniuk, A.; Karcz, W. Single and combined effects of Cd and Pb on the growth, medium pH, membrane potential and metal contents in maize (Zea mays L.) coleoptile segments. Ecotoxicol. Environ. Saf. 2018, 161, 8–16. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Wang, G.; Xu, L.; Shen, Z. Cadmium-induced accumulation of hydrogen peroxide in the leaf apoplast of Phaseolus aureus and Vicia sativa and the roles of different antioxidant enzymes. J. Hazard. Mater. 2009, 168, 76–84. [Google Scholar] [CrossRef]
- Erdei, S. Heavy metal induced physiological changes in the antioxidative response system. Acta Biol. Szeged. 2002, 46, 89–90. [Google Scholar]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Santos, L.; Batista, B.; Lobato, A. Brassinosteroids mitigate cadmium toxicity in cowpea plants. Photosynthetica 2018, 56, 591–605. [Google Scholar] [CrossRef]
- Agrawal, S.; Mishra, S. Effects of supplemental ultraviolet-B and cadmium on growth, antioxidants and yield of Pisum sativum L. Ecotoxicol. Environ. Saf. 2009, 72, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shafi, M.; Wang, Y.; Wu, J.; Ye, Z.; Liu, C.; Zhong, B.; Guo, H.; He, L.; Liu, D. Organic acid compounds in root exudation of Moso Bamboo (Phyllostachys pubescens) and its bioactivity as affected by heavy metals. Environ. Sci. Pollut. Res. 2016, 23, 20977–20984. [Google Scholar] [CrossRef] [PubMed]
- Saffari, V.R.; Saffari, M. Effects of EDTA, citric acid, and tartaric acid application on growth, phytoremediation potential, and antioxidant response of Calendula officinalis L. in a cadmium-spiked calcareous soil. Int. J. Phytoremediat. 2020, 22, 1204–1214. [Google Scholar] [CrossRef] [PubMed]






| Characteristics | Soil Analysis/kg |
|---|---|
| Soil Texture | Slightly loamy |
| pH | 8.06 |
| Electrical Conductivity (dS/m) | 0.8 |
| Soil Moisture Content % | 1.001 |
| Cadmium (mg/kg) | 0.37 |
| Copper (mg/kg) | 0.22 |
| Lead (mg/kg) | 0.05 |
| Zinc (mg/kg) | 0.11 |
| Treatment | Cadmium Concentration | Citric Acid |
|---|---|---|
| Control | 0 | − |
| T1 | 0 | + |
| T2 | 100 mg/kg | − |
| T3 | 100 mg/kg | + |
| T4 | 200 mg/kg | − |
| T5 | 200 mg/kg | + |
| Chlorophyll Contents (mg.g−1 FW) | |||||
|---|---|---|---|---|---|
| Treatments | Chlorophyll a | Chlorophyll b | Total Chlorophylls | Chlorophyll a/b | Carotenoids |
| C | 86.46 ± 0.03 c | 20.4 ± 0.03 c | 106.25 ± 0.02 c | 4.25 ± 0.04 a | 16.03 ± 0.01 b |
| T1 | 144.6 ± 0.04 a | 58.5 ± 0.00 a | 201.92 ± 0.03 a | 2.49 ± 0.03 bc | 17.25 ± 0.00 b |
| T2 | 65.1 ± 0.01 d | 16.6 ± 0.02 c | 81.24 ± 0.01 e | 3.93 ± 0.03 a | 12.54 ± 0.04 c |
| T3 | 113.47 ± 0.03 b | 32.99 ± 0.04 b | 145.61 ± 0.04 b | 3.45 ± 0.01 ab | 34.62 ± 0.02 a |
| T4 | 48.93 ± 0.00 e | 15.8 ± 0.01 c | 64.34 ± 0.00 f | 3.28 ± 0.05 ab | 8.29 ± 0.03 d |
| T5 | 64.85 ± 0.01 d | 32.48 ± 0.01 b | 96.71 ± 0.03 d | 2.02 ± 0.01 c | 8.70 ± 0.02 d |
| Dry Weight (g.plant−1) | Cd Concentration (mg·kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | TI | TF | BAC | BCF | |
| C | 0.5 ± 0.03 b | 0.33 ± 0.01 d | 0.01 ± 0.02 e | 0.01 ± 0.01 d | N.d | N.d | N.d | N.d |
| T1 | 0.4 ± 0.04 c | 0.34 ± 0.03 d | 0.01 ± 0.03 e | 0.01 ± 0.03 d | N.d | N.d | N.d | N.d |
| T2 | 0.43 ± 0.03 c | 0.53 ± 0.05 b | 1.5 ± 0.04 d | 2.43 ± 0.02 c | 0.979518 | 0.61 | 15% | 24% |
| T3 | 0.58 ± 0.01 a | 0.72 ± 0.04 a | 2.1 ± 0.01 c | 2.5 ± 0.02 c | 1.46988 | 0.84 | 21% | 25% |
| T4 | 0.13 ± 0.04 e | 0.18 ± 0.04 e | 2.7 ± 0.02 b | 3.42 ± 0.03 b | 0.46988 | 1.26 | 17% | 13% |
| T5 | 0.28 ± 0.05 d | 0.36 ± 0.03 c | 3.4 ± 0.05 a | 3.9 ± 0.04 a | 1.113253 | 1.14 | 19% | 17% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.A.; Wang, T.; Nisa, Z.U.; Alnusairi, G.S.H.; Shi, F. Insights into Cadmium-Induced Morphophysiological Disorders in Althea rosea Cavan and Its Phytoremediation through the Exogeneous Citric Acid. Agronomy 2022, 12, 2776. https://doi.org/10.3390/agronomy12112776
Khan AA, Wang T, Nisa ZU, Alnusairi GSH, Shi F. Insights into Cadmium-Induced Morphophysiological Disorders in Althea rosea Cavan and Its Phytoremediation through the Exogeneous Citric Acid. Agronomy. 2022; 12(11):2776. https://doi.org/10.3390/agronomy12112776
Chicago/Turabian StyleKhan, Amir Abdullah, Tongtong Wang, Zaib Un Nisa, Ghalia S. H. Alnusairi, and Fuchen Shi. 2022. "Insights into Cadmium-Induced Morphophysiological Disorders in Althea rosea Cavan and Its Phytoremediation through the Exogeneous Citric Acid" Agronomy 12, no. 11: 2776. https://doi.org/10.3390/agronomy12112776