Ghanaian Cocoa (Theobroma cacao L.) Bean Shells Coproducts: Effect of Particle Size on Chemical Composition, Bioactive Compound Content and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
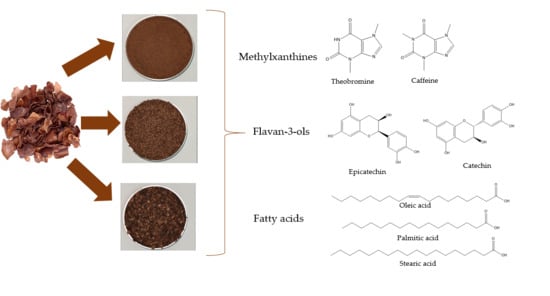
2.2. Preparation of Cocoa Bean Shells Flours with Different Particle Sizes
2.3. Chemical Composition
2.4. Organic Acid and Sugar Content
2.4.1. Extraction of Organic Acid and Sugars
2.4.2. High Performance Liquid Chromatography Analysis
2.5. Water Activity, pH and Color Analysis
2.6. Techno-Functional Properties
2.7. Fatty Acid Profile
2.8. Polyphenolic Profile
2.8.1. Extract Preparation
2.8.2. High Performance Liquid Chromatography Analysis
2.9. Methylxanthines Determination
2.10. Antioxidant Activity
2.11. Statistical Assay
3. Results
3.1. Chemical Composition
3.2. Organic Acid and Sugars
3.3. Physico-Chemical and Techno-Functional Properties
3.4. Fatty Acid Profile
3.5. Polyphenolic Profile
3.6. Methylxanthines Content
3.7. Antioxidant Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ahmad, T.; Aadil, T.R.M.; Ahmed, H.; Rahman, U.; Soares, B.C.V.; Souza, S.L.Q.; Pimentel, T.C.; Scudino, H.; Guimarães, J.T.; Esmerino, E.A.; et al. Treatment and utilization of dairy industrial waste: A review. Trends Food Sci. Technol. 2019, 88, 361–372. [Google Scholar] [CrossRef]
- International Cocoa Organization. Production of Cocoa Beans. ICCO Quarterly Bulletin of Cocoa Statistics, XLVI. 2020. Available online: https://www.icco.org/wp-content/uploads/QBCS-ORDER-FORM-2020.pdf (accessed on 11 November 2020).
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Cocoa bean shell waste valorisation; extraction from lab to pilot-scale cavitational reactors. Food Res. Int. 2019, 115, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, C.; Viera-Alcaide, I.; Sillero, A.M.M.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Bioactive compounds in Mexican genotypes of cocoa cotyledon and husk. Food Chem. 2018, 240, 831–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, I.D.; Gomes Okiyama, D.C.; da Costa Rodrigues, C.E. Simultaneous green extraction of fat and bioactive compounds of cocoa shell and protein fraction functionalities evaluation. Food Res. Int. 2020, 137, 109622. [Google Scholar] [CrossRef]
- Quelal-Vásconez, M.A.; Lerma-García, M.J.; Pérez-Esteve, E.; Arnau-Bonachera, A.; Barat, J.M.; Talens, P. Changes in methylxanthines and flavanols during cocoa powder processing and their quantification by near-infrared spectroscopy. LWT Food Sci. Technol. 2020, 117, 108598. [Google Scholar] [CrossRef]
- Mellinas, A.C.; Jiménez, A.; Garrigós, M.C. Optimization of microwave-assisted extraction of cocoa bean shell waste and evaluation of its antioxidant, physicochemical and functional properties. LWT Food Sci. Technol. 2020, 127, 109361. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Navarro, S.L.B.; Rodrigues, C.E.C. Cocoa shell and its compounds: Applications in the food industry. Trends Food Sci. Technol. 2017, 63, 103–112. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor chemistry of cocoa and cocoa products—An overview. Comp. Rev. Food Sci Food Safety 2016, 15, 73–91. [Google Scholar] [CrossRef]
- Bortolini, C.; Patrone, V.; Puglisi, E.; Morelli, L. Detailed analyses of the bacterial populations in processed cocoa beans of different geographic origin, subject to varied fermentation conditions. Int. J. Food Microbiol. 2016, 236, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.A.; Mahar, I.; Memon, R.; Soomro, S.; Harnly, J.; Memon, N.; Bhangar, M.I.; Luthria, D.L. Impact of flour particle size on nutrient and phenolic acid composition of commercial wheat varieties. J. Food Compo. Anal. 2020, 86, 103358. [Google Scholar] [CrossRef]
- Rodriguez, I.F.; Pérez, M.J.; Cattaneo, F.; Zampini, I.C.; Cuello, A.S.; Mercado, M.I.; Ponessa, G.; Isla, M.I. Morphological, histological, chemical and functional characterization of Prosopis alba flours of different particle sizes. Food Chem. 2019, 274, 583–591. [Google Scholar] [CrossRef]
- Lucas-González, R.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Effect of particle size on phytochemical composition and antioxidant properties of two persimmon flours from Diospyros kaki Thunb. vars. “Rojo Brillante“ and “Triumph“ co-products. J. Sci. Food Agric. 2018, 98, 504–510. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Washington, DC, USA, 2007. [Google Scholar]
- Melgarejo-Sánchez, P.; Martínez, J.J.; Legua, L.; Martínez, R.; Hernández, F.; Melgarejo, P. Quality, antioxidant activity and total phenols of six Spanish pomegranates clones. Sci. Hortic. 2015, 182, 65–72. [Google Scholar] [CrossRef]
- Chau, C.F.; Huang, Y.L. Comparison of the chemical composition and physicochemical properties of different fibres prepared from peel of Citrus sinensis L. cv. Liucheng. J. Agric. Food Chem. 2003, 51, 2615–2618. [Google Scholar] [CrossRef]
- Golay, P.A.; Moulin, J. Determination of labeled fatty acids content in milk products, infant formula, and adult/pediatric nutritional formula by capillary gas chromatography: Collaborative study, Final Action 2012.13. J. AOAC Int. 2016, 99, 210–222. [Google Scholar] [CrossRef] [Green Version]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui [Aristotelia chilensis (Molina) Stuntz] a Chilean blackberry. J. Sci. Food Agric. 2016, 96, 4235–4242. [Google Scholar] [CrossRef] [PubMed]
- Mpofu, A.; Sapirstein, H.D.; Beta, T. Genotype and environmental variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. J. Agric. Food Chem 2006, 54, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Gullón, B.; Pintado, M.E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. In Vitro gastrointestinal digestion of pomegranate peel (Punica granatum) flour obtained from co-products: Changes in the antioxidant potential and bioactive compounds stability. J. Funct. Foods 2015, 19, 617–628. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activity of products of browning reaction prepared from glucosamine. Jap J. Nut 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Mahdavi, B.; Yaacob, W.A.; Din, L.B. Chemical composition, antioxidant, and antibacterial activity of essential oils from Etlingera sayapensis A.D. Poulsen & Ibrahim. Asian Pac. J. Trop. Med. 2017, 10, 819–826. [Google Scholar] [CrossRef]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of cocoa (Theobroma cacao L.) co-products. Food Res. Int. 2012, 49, 39–45. [Google Scholar] [CrossRef]
- Agus, B.A.P.; Mohamad, N.N.; Hussain, N. Composition of unfermented, unroasted, roasted cocoa beans and cocoa shells from Peninsular Malaysia. J. Food Meas. 2018, 12, 2581–2589. [Google Scholar] [CrossRef]
- Luthria, D.L.; Noel, K.; Vinjamoori, D. Impact of sample preparation on the determination of crude fat content in corn. J. Am. Oil Chem. Soc. 2004, 81, 999–1004. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Mateus-Reguengo, L.; Bertolino, M.; Stévigny, C.; Zeppa, G.E. effects of particle size and extraction methods on cocoa bean shell functional beverage. Nutrients 2019, 11, 867. [Google Scholar] [CrossRef] [Green Version]
- Lecumberri, E.; Mateos, R.; Izquierdo-Pulido, M.; Rupérez, P.; Goya, L.; Bravo, L. Dietary fibre composition, antioxidant capacity and physico-chemical properties of a fibre-rich product from cocoa (Theobroma cacao L.). Food Chem. 2007, 104, 948–954. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-Lopez, J.; Pérez-Alvarez, J.A. Dietary fiber as an ingredient for the meat industry. In Dietary Fiber, Sources, Properties and Their Relationship to Health; Nova: Hauppauge, NY, USA, 2013. [Google Scholar]
- Shah, B.R.; Li, B.; Al Sabbah, H.; Xu, W.; Mráz, J. Effects of prebiotic dietary fibers and probiotics on human health: With special focus on recent advancement in their encapsulated formulations. Trends Food Sci. Technol. 2020, 102, 178–192. [Google Scholar] [CrossRef]
- Hamdouche, Y.; Meile, J.C.; Lebrun, M.; Guehi, T.; Boulanger, R.; Teyssier, C.; Didier Montet, D. Impact of turning, pod storage and fermentation time on microbial ecology and volatile composition of cocoa beans. Food Res. Int. 2019, 119, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Redgwell, R.; Trovato, V.; Merinat, S.; Curti, D.; Hediger, S.; Manez, A. Dietary fibre in cocoa shell: Characterisation of component polysaccharides. Food Chem. 2003, 81, 103–112. [Google Scholar] [CrossRef]
- Jokíc, S.; Gagíc, T.; Knez, Z.; Šubaríc, D.; Škerget, M. Separation of active compounds from food by-product (cocoa shell) using subcritical water extraction. Molecules 2018, 23, 1408. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Chen, J.; Ma, J.; Sun, H.; Yuan, Y.; Ju, Q.; Teng, Y.; Yang, M.; Li, W.; Fujita, K.; et al. Effects of different milling methods on physicochemical properties of common buckwheat flour. LWT Food Sci. Technol. 2018, 92, 220–226. [Google Scholar] [CrossRef]
- Hidalgo, A.; Fongaro, L.; Brandolini, A. Wheat flour granulometry determines colour perception. Food Res. Int. 2014, 64, 363–370. [Google Scholar] [CrossRef]
- Jacobs, P.J.; Hemdane, S.; Dornez, E.; Delcour, J.A.; Courtin, C.M. Study of hydration properties of wheat bran as a function of particle size. Food Chem 2015, 179, 296–304. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Pérez-Álvarez, J.A.; Fernández-López, J. Evaluation of particle size influence on proximate composition, physicochemical, techno-functional and physio-functional properties of flours obtained from persimmon (Diospyros kaki Trumb.) coproducts. Plant. Foods Hum. Nutr. 2017, 72, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ancona, D.B.; Mercado, G.P.; Ordoñez, Y.M.; Blanco, S.F. Physicochemical characterization lima bean (Phaseolus lunatus) and Jack bean (Canavalia ensiformis) fibrous residues. Food Chem 2004, 84, 287–295. [Google Scholar] [CrossRef]
- Noort, M.W.; van Haaster, D.; Hemery, Y.; Schols, H.A.; Hamer, R.J. The effect of particle size of wheat bran fractions on bread quality: Evidence for fibre-protein interactions. J. Cereal Sci. 2010, 52, 59–64. [Google Scholar] [CrossRef]
- Lessa, O.A.; Reis, N.S.; Leite, S.G.F.; Gutarra, M.L.E.; Souza, A.O.; Gualberto, S.A.; de Oliveira, J.R.; Aguilar-Oliveira, E.; Franco, M. Effect of the solid state fermentation of cocoa shell on the secondary metabolites, antioxidant activity, and fatty acids. Food Sci. Biotechnol. 2018, 27, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Zaiter, A.; Becker, L.; Baudelaire, E.; Dicko, A. Optimum polyphenol and triterpene contents of Hedera helix (L.) and Scrophularia nodosa (L.): The role of powder particle size. Microchem. J. 2018, 137, 168–173. [Google Scholar] [CrossRef]
- Meullemiestre, A.; Petitcolas, E.; Maache-Rezzoug, Z.; Chemat, F.; Rezzoug, S.A. Impact of ultrasound on solid-liquid extraction of phenolic compounds from maritime pine sawdust waste. Kinetics, optimization and large scale experiments. Ultrasonics Sonochem. 2016, 28, 230–239. [Google Scholar] [CrossRef]
- Hernández-Hernández, C.; Morales-Sillero, A.; Fernández-Bolaños, J.; Bermúdez-Oria, A.; Morales, A.A.; Rodríguez-Gutiérrez, G. Cocoa bean husk: Industrial source of antioxidant phenolic extract. J. Sci. Food Agric. 2019, 99, 325–333. [Google Scholar] [CrossRef]
- Papillo, V.A.; Locatelli, M.; Travaglia, F.; Bordiga, M.; Garino, C.; Coïsson, J.D.; Arlorio, M. Cocoa hulls polyphenols stabilized by microencapsulation as functional ingredient for bakery applications. Food Res. Int. 2019, 115, 511–518. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Soares, I.D.; Cuevas, M.S.; Crevelin, E.J.; Moraes, L.A.B.; MaMelo, M.P.; Oliveira, A.L.; Rodrigues, C.E.C. Pressurized liquid extraction of flavanols and alkaloids from cocoa bean shell using ethanol as solvent. Food Res. Int. 2018, 114, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Pereira, L.; Guglielmetti, A.; Zeppa, G. Pulsed electric field assisted extraction of bioactive compounds from cocoa bean shell and coffee silver skin. Food Bioproc. Technol. 2018, 11, 818–835. [Google Scholar] [CrossRef]
- Arlorio, M.; Coïsson, J.D.; Travaglia, F.; Varsaldi, F.; Miglio, G.; Lombardi, G.; Martelli, A. Antioxidant and biological activity of phenolic pigments from Theobroma cacao hulls extracted with supercritical CO2. Food Res. Int. 2005, 38, 1009–1014. [Google Scholar] [CrossRef]


| Moisture | Protein | Fat | Ash | TDF | |
|---|---|---|---|---|---|
| HCBS | 7.05 ± 0.13 a | 15.59 ± 0.22 b | 3.00 ± 0.02 b | 7.09 ± 0.73 a | 65.58 ± 0.53 a |
| ICBS | 6.90 ± 0.36 a | 15.95 ± 0.13 b | 3.60 ± 0.19 b | 7.03 ± 0.65 a | 62.03 ± 0.13 b |
| LCBS | 6.79 ± 0.19 a | 17.13 ± 0.17 a | 5.60 ± 0.24 a | 7.34 ± 0.35 a | 61.18 ± 0.94 b |
| Organic Acids | ||||||||||
| Oxalic Acid | Citric Acid | Succinic Acid | Tartaric Acid | Malic Acid | Acetic Acid | Total | ||||
| HCBS | 1.57 ± 0.07 cB | 1.62 ± 0.13 cB | 2.50 ± 0.41 aA | 2.68 ± 0.02 aA | 2.46 ± 0.14 aA | 1.03 ± 0.01 cC | 11.85 ± 0.23 b | |||
| ICBS | 1.62 ± 0.04 bC | 1.97 ± 0.06 bB | 2.20 ± 0.33 aA | 2.61 ± 0.08 aA | 2.47 ± 0.10 aA | 1.33 ± 0.03 bD | 12.20 ± 0.14 b | |||
| LCBS | 2.33 ± 0.10 aB | 2.76 ± 0.11 aA | 2.06 ± 0.09 aC | 2.79 ± 0.06 aA | 2.67 ± 0.12 aA | 1.72 ± 0.17 aD | 14.34 ± 0.11 a | |||
| Sugars | ||||||||||
| Sucrose | Glucose | Fructose | Arabinose | Total | ||||||
| HCBS | 1.12 ± 0.07 bC | 5.81 ± 0.03 cA | 5.06 ± 0.04 bB | 1.21 ± 0.04 cC | 13.20 ± 0.12 c | |||||
| ICBS | 1.22 ± 0.07 abD | 6.17 ± 0.04 bA | 5.17 ± 0.14 bB | 1.56 ± 0.04 bC | 14.10 ± 0.09 b | |||||
| LCBS | 1.34 ± 0.10 aD | 6.33 ± 0.01 aA | 5.45 ± 0.08 aB | 2.45 ± 0.04 aC | 15.57 ± 0.08 a | |||||
| Physic-Chemical Properties | |||||||
| Color Coordinates | |||||||
| pH | Aw | L* | a* | b* | |||
| HCBS | 5.07 ± 0.03 a | 0.474 ± 0.001 b | 45.97 ± 0.75 c | 5.78 ± 0.19 c | 8.16 ± 0.48 c | ||
| ICBS | 4.93 ± 0.03 b | 0.495 ± 0.006 a | 47.84 ± 1.11 b | 6.53 ± 0.31 b | 9.45 ± 0.55 b | ||
| LCBS | 4.90 ± 0.06 b | 0.501 ± 0.004 a | 50.17 ± 0.31 a | 8.06 ± 0.09 a | 11.84 ± 0.18 a | ||
| Techno-functional properties | |||||||
| WHC | OHC | SWC | |||||
| HCBS | 5.07 ± 0.27 a | 2.74 ± 0.08 a | 3.16 ± 0.05 a | ||||
| ICBS | 4.15 ± 0.19 b | 1.55 ± 0.05 b | 2.49 ± 0.10 b | ||||
| LCBS | 3.30 ± 0.20 c | 1.28 ± 0.08 c | 1.31 ± 0.08 c | ||||
| HCBS | ICBS | LCBS | |
|---|---|---|---|
| C10:0 | 1.20 ± 0.12 c | 2.99 ± 0.09 b | 4.48 ± 0.38 a |
| C12:0 | 1.72 ± 0.21 c | 8.14 ± 0.37 a | 3.92 ± 0.11 b |
| C13:0 | 4.20 ± 0.25 a | N.D. | N.D. |
| C14:0 | 18.28 ± 0.15 c | 20.69 ± 0.98 b | 29.44 ± 2.36 a |
| C15:0 | 1.94 ± 0.09 c | 2.60 ± 0.17 b | 3.70 ± 0.07 a |
| C16:0 | 736.82 ± 3.58 c | 882.50 ± 4.72 b | 1371.21 ± 8.74 a |
| C16:1 | 31.82 ± 0.96 c | 28.42 ± 0.14 b | 51.49 ± 1.58 a |
| C17:0 | 9.22 ± 0.41 c | 10.96 ± 0.32 b | 15.88 ± 0.45 a |
| C17:1 | 2.56 ± 0.18 c | 3.96 ± 0.17 a | 3.06 ± 0.09 b |
| C18:0 | 834.96 ± 4.89 c | 1054.84 ± 10.87 b | 1618.40 ± 12.17 a |
| C18:1 (n9) | 1040.76 ± 5.47 c | 1193.81 ± 9.56 b | 1855.52 ± 11.23 a |
| C18:2 (n6,9) | 220.48 ± 2.47 c | 268.65 ± 5.63 b | 442.40 ± 8.96 a |
| C18:3 (n3,6,9) | 19.48 ± 0.57 c | 25.41 ± 0.47 b | 41.24 ± 1.59 a |
| C18:3 (n6,9,12) | 1.04 ± 0.07 c | 3.70 ± 0.03 b | N.D. |
| C20:0 | 34.67 ± 0.68 c | 45.49 ± 0.89 b | 70.79 ± 3.56 aD |
| C20:1 | 5.15 ± 0.74 a | 3.13 ± 0.12 c | 7.84 ± 0.75 a |
| C20:2 (n11,14) | 0.77 ± 0.07 b | N.D. | 4.07 ± 0.58 a |
| C20:3 (n8,11,14) | 0.47 ± 0.08 c | 3.05 ± 0.07 b | 4.62 ± 0.96 a |
| C20:3 (n11,14,17) | 2.87 ± 0.14 c | 1.96 ± 0.08 c | 4.33 ± 0.63 a |
| C20:5 (n5,8,11,14,17) | 1.30 ± 0.02 c | N.D. | N.D. |
| C22:0 | 13.19 ± 0.07 c | 19.00 ± 0.87 b | 31.07 ± 1.73 a |
| C22:2 | 0.35 ± 0.05 c | N.D. | N.D. |
| C22:5 | 3.96±0.04 c | 4.05 ± 0.11 b | 9.41 ± 0.68 a |
| C24:0 | 12.71±0.21 c | 16.64 ± 0.56 b | 27.32 ± 1.09 a |
| ΣSFA | 1668.91±5.69 c | 2063.85 ± 5.7 b | 3176.21 ± 6.91 a |
| ΣMUFA | 1080.29±4.87 c | 1229.31 ± 6.89 b | 1917.91 ± 10.89 a |
| ΣPUFA | 250.72±3.74 c | 306.82 ± 3.96 b | 506.07 ± 4.87 a |
| ΣPUFA/ΣSFA | 0.15±0.03 a | 0.15± 0.04 a | 0.16±0.04 a |
| Compound | HCBS | ICBS | LCBS | |
|---|---|---|---|---|
| Catechin | Free | 1966.89 ± 54.98 c | 2493.37 ± 32.25 b | 4211.82 ± 29.81 a |
| Bound | 143.91 ± 9.76 c | 224.12 ± 18.23 b | 354.76 ± 8.25 a | |
| TOTAL | 2110.88 ± 12.59 | 2717.49 ± 28.96 | 4566.58 ± 16.96 | |
| Epicatechin | Free | 4467.41 ± 31.99 c | 4815.92 ± 29.76 b | 6087.49 ± 25,87 a |
| Bound | 95.28 ± 7.34 c | 114.59 ± 8.76 b | 249.69 ± 12.92 a | |
| TOTAL | 4562.69 ± 16.36 | 4930.51 ± 17.96 | 6337.18 ± 17.82 | |
| Epicatechin derivative | Free | 392.09 ± 8.63 c | 442.24 ± 9.14 b | 482.85 ± 11.67 a |
| Bound | N.D. | N.D. | N.D. | |
| TOTAL | 392.09 ± 8.63 | 442.24 ± 9.14 | 482.85 ± 11.67 | |
| Quercetin | Free | 128.34 ± 8.34 c | 167.69± 10.89 b | 227.15 ± 8.52 a |
| Bound | 2.40 ± 0.12 c | 4.53± 0.22 b | 6.98 ± 0.37 a | |
| TOTAL | 130.74 ± 3.57 | 172.22 ± 4.23 | 234.13 ± 5.36 | |
| Quercetin-3-O-glucosie | Free | 100.35 ± 1.59 c | 123.07 ±3.82 b | 289.61 ± 4.36 a |
| Bound | 3.35 ± 0.14 c | 4.09± 0.27 b | 7.33 ± 0.48 a | |
| TOTAL | 103.70 ± 0.85 | 127.16 ± 0.98 | 296.94 ± 2.12 | |
| Quercetin-3-O-rutinosie | Free | 92.70 ± 3.87 c | 137.89 ± 8.56 b | 237.53 ± 9.72 a |
| Bound | 1.48 ± 0.09 c | 1.61 ± 0.08 b | 3.73 ± 0.12 a | |
| TOTAL | 94.18 ± 1.14 | 139.50 ± 3.14 | 241.26 ± 5.21 | |
| Protocatechuic acid | Free | N.D. | N.D. | N.D. |
| Bound | 80.53 ± 1.92 c | 100.79 ± 2.98 b | 148.93 ± 1.23 a | |
| TOTAL | 80.53 ± 1.92 | 100.79 ± 2.98 | 148.93 ± 1.23 |
| DPPH (mg TE/g) | ABTS (mg TE/g) | FRAP (mg TE/g) | FIC (mg EDTAE/g) | |
|---|---|---|---|---|
| HCBS | 2.35 ± 0.11 c | 3.39 ± 0.20 c | 3.84 ± 0.02 c | 0.28 ± 0.01 c |
| ICBS | 4.21 ± 0.05 b | 6.49 ± 0.44 b | 5.30 ± 0.02 b | 0.40 ± 0.03 b |
| LCBS | 5.53 ± 0.04 a | 11.55 ± 0.46 a | 7.62 ± 0.04 a | 0.54 ± 0.02 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botella-Martínez, C.; Lucas-Gonzalez, R.; Ballester-Costa, C.; Pérez-Álvarez, J.Á.; Fernández-López, J.; Delgado-Ospina, J.; Chaves-López, C.; Viuda-Martos, M. Ghanaian Cocoa (Theobroma cacao L.) Bean Shells Coproducts: Effect of Particle Size on Chemical Composition, Bioactive Compound Content and Antioxidant Activity. Agronomy 2021, 11, 401. https://doi.org/10.3390/agronomy11020401
Botella-Martínez C, Lucas-Gonzalez R, Ballester-Costa C, Pérez-Álvarez JÁ, Fernández-López J, Delgado-Ospina J, Chaves-López C, Viuda-Martos M. Ghanaian Cocoa (Theobroma cacao L.) Bean Shells Coproducts: Effect of Particle Size on Chemical Composition, Bioactive Compound Content and Antioxidant Activity. Agronomy. 2021; 11(2):401. https://doi.org/10.3390/agronomy11020401
Chicago/Turabian StyleBotella-Martínez, Carmen, Raquel Lucas-Gonzalez, Carmen Ballester-Costa, José Ángel Pérez-Álvarez, Juana Fernández-López, Johannes Delgado-Ospina, Clemencia Chaves-López, and Manuel Viuda-Martos. 2021. "Ghanaian Cocoa (Theobroma cacao L.) Bean Shells Coproducts: Effect of Particle Size on Chemical Composition, Bioactive Compound Content and Antioxidant Activity" Agronomy 11, no. 2: 401. https://doi.org/10.3390/agronomy11020401