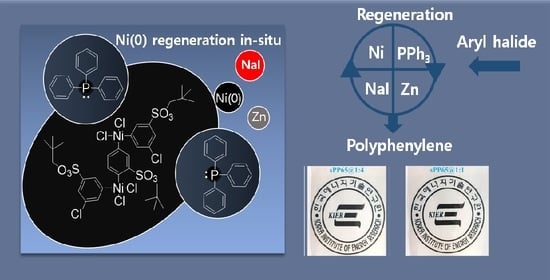
Synthesis of Sulfonated Polyphenylene Block Copolymers via In Situ Generation of Ni(0)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Neopentyl 2,5-Dichlorobenzenesulfonate and Neopentyl 3,5-Dichlorobenzenesulfonate
2.3. Synthesis of Hydrophobic Cl-End-Capped OAEK Oligomer
2.4. Synthesis of sPP Block Copolymers via Ni(II)-Catalyzed Colon’s Cross-Coupling Reaction
2.5. Synthesis of sPP Block Copolymers via Ni(0)-Catalyzed Colon’s Cross-Coupling Reaction
2.6. Deprotection and Protonation of sPP Block Copolymers
2.7. Preparation of sPP Block Copolymer Membranes
2.8. Molecular Weight Measurements
2.9. Structural Analysis
2.10. Water Uptake, Swelling Ratio, and Conductivity Measurements for Membranes
3. Results and Discussion
3.1. Synthesis of Hydrophobic Cl-End-Capped OAEK Oligomer
3.2. Block Copolymer Synthesis Using NiBr2(PPh3)2 with the Reducing Metal Zn
3.2.1. Effect of the Equivalents of the Catalyst and Monomers on the Polymer Molecular Weight
3.2.2. Role of Zn and NaI in the Polymerization Reaction
3.2.3. Block Copolymer Synthesis Using Ni(COD)2
3.3. Structural Characterization of the Synthesized Block Copolymers
3.4. Fabrication of PEMs from the Synthesized sPP Block Copolymers
3.5. Ion Exchange Capacity, Proton Conductivity, Water Uptake, and Dimension Stability of Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Oshima, T.; Yoshizawa-Fujita, M.Y.; Takeoka, Y.; Rikukawa, M. Use of a High-Performance Poly(p-Phenylene)-Based Aromatic Hydrocarbon Ionomer with Superacid Groups in Fuel Cells under Low Humidity Conditions. ACS Omega 2016, 1, 939–942. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yu, D.M.; Kim, T.H.; Yoon, S.J.; Hong, Y.T. Multiblock Copolymers Based on Poly(p-Phenylene)s with Excellent Durability and Fuel Cell Performance. J. Membr. Sci. 2015, 492, 209–219. [Google Scholar] [CrossRef]
- Wijaya, F.; Woo, S.; Lee, H.; Nugraha, A.F.; Shin, D.; Bae, B. Sulfonated Poly(Phenylene-Co-Arylene Ether Sulfone) Multiblock Membranes for Application in High-Performance Fuel Cells. J. Membr. Sci. 2022, 645, 120203. [Google Scholar] [CrossRef]
- Liu, F.; Miyatake, K. Well-Designed Polyphenylene PEMs with High Proton Conductivity and Chemical and Mechanical Durability for Fuel Cells. J. Mater. Chem. A 2022, 10, 7660–7667. [Google Scholar] [CrossRef]
- Nederstedt, H.; Jannasch, P. Poly(p-Terphenyl Alkylene)s Grafted with Highly Acidic Sulfonated Polypentafluorostyrene Side Chains for Proton Exchange Membranes. J. Membr. Sci. 2022, 647, 120270. [Google Scholar] [CrossRef]
- Ge, X.; Zhang, F.; Wu, L.; Yang, Z.; Xu, T. Current Challenges and Perspectives of Polymer Electrolyte Membranes. Macromolecules 2022, 55, 3773–3787. [Google Scholar] [CrossRef]
- Shin, H.Y.; Cha, M.S.; Hong, S.H.; Kim, T.H.; Yang, D.S.; Oh, S.G.; Lee, J.Y.; Hong, Y.T. Poly(p-Phenylene)-Based Membrane Materials with Excellent Cell Efficiencies and Durability for Use in Vanadium Redox Flow Batteries. J. Mater. Chem. A 2017, 5, 12285–12296. [Google Scholar] [CrossRef]
- Hong, S.H.; Cha, M.S.; Hong, S.K.; Oh, S.G.; Lee, J.Y. Structural Effect of the Hydrophobic Block on the Chemical Stability of Ion-Conducting Multiblock Copolymers for Flow Battery. ACS Sustain. Chem. Eng. 2019, 7, 17088–17099. [Google Scholar] [CrossRef]
- Chen, S.; Hara, R.; Chen, K.; Zhang, X.; Endo, N.; Higa, M.; Okamoto, K.; Wang, L. Poly(Phenylene) Block Copolymers Bearing Tri-Sulfonated Aromatic Pendant Groups for Polymer Electrolyte Fuel Cell Applications. J. Mater. Chem. A 2013, 1, 8178–8189. [Google Scholar] [CrossRef]
- Elabd, Y.A.; Hickner, M.A. Block Copolymers for Fuel Cells. Macromolecules 2011, 44, 1–11. [Google Scholar] [CrossRef]
- Takeoka, Y.; Umezawa, K.; Oshima, T.; Yoshida, M.; Yoshizawa-Fujita, M.Y.; Rikukawa, M. Synthesis and Properties of Hydrophilic–Hydrophobic Diblock Copolymer Ionomers Based on Poly(p-Phenylene)s. Polym. Chem. 2014, 5, 4132–4140. [Google Scholar] [CrossRef]
- Tonozuka, I.; Yoshida, M.; Kaneko, K.; Takeoka, Y.; Rikukawa, M. Considerations of Polymerization Method and Molecular Weight for Proton-Conducting Poly(p-Phenylene) Derivatives. Polymer 2011, 52, 6020–6028. [Google Scholar] [CrossRef]
- Nugraha, A.F.; Kim, S.; Wijaya, F.; Bae, B.; Shin, D. Synthetic Approaches for Poly(Phenylene) Block Copolymers via Nickel Coupling Reaction for Fuel Cell Applications. Polymers 2020, 12, 1614. [Google Scholar] [CrossRef]
- Miyake, J.; Taki, R.; Mochizuki, T.; Shimizu, R.; Akiyama, R.; Uchida, M.; Miyatake, K. Design of Flexible Polyphenylene Proton-Conducting Membrane for Next-Generation Fuel Cells. Sci. Adv. 2017, 3, eaao0476. [Google Scholar] [CrossRef] [Green Version]
- Hosaka, I.; Sawano, T.; Kimura, T.; Matsumoto, A.; Miyake, J.; Miyatake, K. Differences in the Synthetic Method Affected Copolymer Sequence and Membrane Properties of Sulfonated Polymers. Bull. Chem. Soc. Jpn. 2020, 93, 393–398. [Google Scholar] [CrossRef]
- Wang, Y.; Quirk, R.P. Synthesis and Characterization of Poly(Benzoyl-1,4-Phenylene)s. 2. Catalyst Coligand Effects on Polymer Properties. Macromolecules 1995, 28, 3495–3501. [Google Scholar] [CrossRef]
- Colon, I.; Kelsey, D.R. Coupling of Aryl Chloride by Nickel and Reducing Metal. J. Org. Chem. 1985, 51, 2627–2637. [Google Scholar] [CrossRef]
- Lee, H.S.; Roy, A.; Lane, O.; Dunn, S.; McGrath, J.E. Hydrophilic–Hydrophobic Multiblock Copolymers Based on Poly(Arylene Ether Sulfone) via Low-Temperature Coupling Reactions for Proton Exchange Membrane Fuel Cells. Polymer 2008, 49, 715–723. [Google Scholar] [CrossRef]
- Shin, D.; Nugraha, A.F.; Wijaya, F.; Lee, S.; Kim, E.; Choi, J.; Kim, H.J.; Bae, B. Synthetic Approaches for Advanced Multi-block Anion Exchange Membranes. RSC Adv. 2019, 9, 21106–21115. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sheares, V.V. Novel Poly[3-(p-Substituted)Benzoyl-2,5-Thiophenes] via Nickel(0)-Catalyzed Coupling Polymerization. Macromolecules 1998, 31, 6769–6775. [Google Scholar] [CrossRef]
- Ueda, M.; Ito, T. Synthesis of Aromatic Poly(Ether Sulfone)s by Nickel-Catalyzed Coupling Polymerization of Aromatic Dichlorides. Polym. J. 1991, 23, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Colon, I.; Kwiatkowski, G.T. High Molecular Weight Aromatic Polymers by Nickel Coupling of Aryl Polychlorides. J. Polym. Sci. A Polym. Chem. 1990, 28, 367–383. [Google Scholar] [CrossRef]
- Liu, F.; Ahn, J.; Miyake, J.; Miyatake, K. Poly(para-Phenylene) Ionomer Membranes: Effect of Methyl and Trifluoromethyl Substituents. Polym. Chem. 2021, 12, 6101–6109. [Google Scholar] [CrossRef]
- Yang, K.; Li, X.; Guo, J.; Zheng, J.; Li, S.; Zhang, S.; Cao, X.; Sherazi, T.A.; Liu, X. Preparation and Properties of Anion Exchange Membranes with Exceptional Alkaline Stable Polymer Backbone and Cation Groups. J. Membr. Sci. 2020, 596, 117720. [Google Scholar] [CrossRef]
- Takagi, K.; Hayama, N.; Inokawa, S. The In Situ-Generated Nickel(0)-Catalyzed Reaction of Aryl Halides with Potassium Iodide and Zinc Powder. Bull. Chem. Soc. Jpn. 1980, 53, 3691–3695. [Google Scholar] [CrossRef] [Green Version]
- Ueda, M.; Ichikawa, F. Synthesis of aromatic poly(ether ketone)s by nickel-catalyzed coupling polymerization of aromatic dichlorides. Macromolecules 1990, 23, 926–930. [Google Scholar] [CrossRef]
- Ghassemi, H.; McGrath, J.E. Synthesis of poly(arylene phosphine oxide) by nickel-catalyzed coupling polymerization. Polymer 1997, 38, 3139–3143. [Google Scholar] [CrossRef]








| Copolymer | Catalyst | Catalyst Equivalents a | Ligand | Ligand Equivalents b | Comonomer Molar Ratio c | Mn (kDa) | Mw (kDa) | PDI | Yield |
|---|---|---|---|---|---|---|---|---|---|
| sPP30@1:1 sPP50@1:1 sPP65@1:1 sPP75@1:1 sPP65@1:4 sPP(COD)@1:1 sPP(COD)@1:4 | NiBr2(PPh3)2 NiBr2(PPh3)2 NiBr2(PPh3)2 NiBr2(PPh3)2 NiBr2(PPh3)2 Ni(COD)2 Ni(COD)2 | 0.030 0.050 0.065 0.075 0.065 1.25 1.25 | PPh3 PPh3 PPh3 PPh3 PPh3 BPy BPy | 10 10 10 10 10 2 2 | 1:1 1:1 1:1 1:1 1:4 1:1 1:4 | 10 17 22 10 30 18 25 | 20 43 55 26 65 48 57 | 1.9 2.5 2.5 2.5 2.2 2.5 2.3 | 76 66 92 72 83 86 81 |
| Copolymer | IEC (meq/g) | Φw (%) 25 °C | Swelling Ratio (%) 25 °C | ||
|---|---|---|---|---|---|
| Feed | Measured a | Δl | Δt | ||
| sPP65@1:1 | 2.50 | 2.39 | 63 | 22 | 34 |
| sPP65@1:4 | 2.50 | 1.84 | 59 | 17 | 30 |
| sPP(COD)@1:1 | 2.50 | 2.66 | 71 | 36 | 36 |
| sPP(COD)@1:4 | 2.50 | 1.87 | 61 | 22 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, V.; Wijaya, F.; Lee, H.; Bae, B.; Shin, D. Synthesis of Sulfonated Polyphenylene Block Copolymers via In Situ Generation of Ni(0). Polymers 2023, 15, 1577. https://doi.org/10.3390/polym15061577
Yadav V, Wijaya F, Lee H, Bae B, Shin D. Synthesis of Sulfonated Polyphenylene Block Copolymers via In Situ Generation of Ni(0). Polymers. 2023; 15(6):1577. https://doi.org/10.3390/polym15061577
Chicago/Turabian StyleYadav, Vikrant, Farid Wijaya, Hyejin Lee, Byungchan Bae, and Dongwon Shin. 2023. "Synthesis of Sulfonated Polyphenylene Block Copolymers via In Situ Generation of Ni(0)" Polymers 15, no. 6: 1577. https://doi.org/10.3390/polym15061577