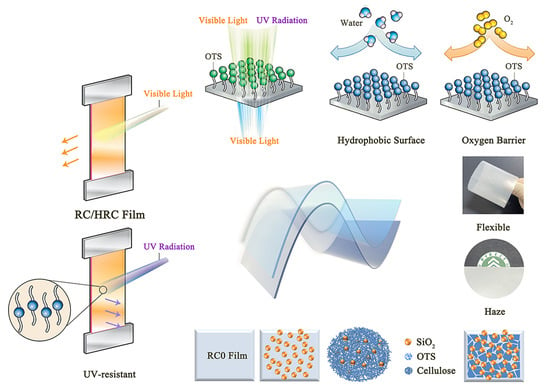
Surface Engineering of Regenerated Cellulose Nanocomposite Films with High Strength, Ultraviolet Resistance, and a Hydrophobic Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. Preparation of Nano-SiO2/Cellulose Solutions
2.2.2. Preparation of Nano-SiO2/Regenerated Cellulose Composite Films
2.2.3. Hydrophobic Modification of Regenerated Cellulose Composite Films
2.2.4. Characterization
3. Results and Discussion
3.1. Morphology and Structure of the Films
3.2. Mechanical Properties and Thermal Stability
3.3. Optical and UV-Blocking Properties of the RC Films and HRC Films
3.4. Oxygen Barrier Properties of the RC and HRC Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olson, E.; Liu, F.; Blisko, J.; Li, Y.; Tsyrenova, A.; Mort, R.; Vorst, K.; Curtzwiler, G.; Yong, X.; Jiang, S. Self-assembly in biobased nanocomposites for multifunctionality and improved performance. Nanoscale Adv. 2021, 3, 4321–4348. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Cuiffi, J.D.; Mathers, R.T. Ranking environmental degradation trends of plastic marine debris based on physical properties and molecular structure. Nat. Commun. 2020, 11, 727. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Huang, J. Cellulose—Rich Nanofiber—Based Functional Nanoarchitectures. Adv. Mater. 2016, 28, 1143–1158. [Google Scholar] [CrossRef]
- Rogina-Car, B.; Budimir, A.; Turcic, V.; Katovic, D. Do multi-use cellulosic textiles provide safe protection against the contamination of sterilized items? Cellulose 2014, 21, 2101–2109. [Google Scholar] [CrossRef]
- He, M.; Xu, M.; Zhang, L. Controllable stearic acid crystal induced high hydrophobicity on cellulose film surface. ACS Appl. Mater. Inter. 2013, 5, 585–591. [Google Scholar] [CrossRef]
- Li, J.; Nawaz, H.; Wu, J.; Zhang, J.; Wan, J.; Mi, Q.; Yu, J.; Zhang, J. All-cellulose composites based on the self-reinforced effect. Compos. Commun. 2018, 9, 42–53. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Jiang, L.; Yao, X. Bioinspired Surfaces with Superwettability: New Insight on Theory, Design, and Applications. Chem. Rev. 2015, 115, 8230–8293. [Google Scholar] [CrossRef] [PubMed]
- Taajamaa, L.; Kontturi, E.; Laine, J.; Rojas, O.J. Bicomponent fibre mats with adhesive ultra-hydrophobicity tailored with cellulose derivatives. J. Mater. Chem. 2012, 22, 12072–12082. [Google Scholar] [CrossRef]
- Gas-phase surface modification of cellulose microfibrils and whiskers. Biomacromolecules 2009, 10, 2144–2151. [CrossRef]
- Wang, J.; Somasundaran, P. Mechanisms of ethyl(hydroxyethyl) cellulose–solid interaction: Influence of hydrophobic modification—ScienceDirect. J. Colloid Interface Sci. 2006, 293, 322–332. [Google Scholar] [CrossRef]
- Ding, B.; Li, C.; Hotta, Y.; Kim, J.; Shiratori, S. Conversion of an electrospun nanofibrous cellulose acetate mat from a super-hydrophilic to super-hydrophobic surface. Jpn. J. Crop Sci. 2006, 17, 4332. [Google Scholar] [CrossRef]
- Cheng, H.; Gu, B.; Pennefather, M.P.; Nguyen, T.X.; Phan-Thien, N.; Duong, H.M. Cotton aerogels and cotton-cellulose aerogels from environmental waste for oil spillage cleanup. Mater. Des. 2017, 130, 452–458. [Google Scholar] [CrossRef]
- Hays, H.L.; Spiller, H. Fluoropolymer-associated illness. Clin. Toxicol. 2014, 52, 848–855. [Google Scholar] [CrossRef]
- Smith, D.W.; Iacono, S.T.; Iyer, S.S. Handbook of Fluoropolymer Science and Technology (Smith/Handbook). In Interfacial Response of Semifluorinated Multi-Block Copolymers; Wiley Online Library: Hoboken, NJ, USA, 2014; pp. 43–56. [Google Scholar] [CrossRef]
- Kumar, A.; Staněk, K.; Ryparová, P.; Hajek, P.; Tywoniak, J. Hydrophobic treatment of wood fibrous thermal insulator by octadecyltrichlorosilane and its influence on hygric properties and resistance against moulds. Compos. Part B Eng. 2016, 106, 285–293. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, T.; Xiao, S.; He, H.; Yang, J.; Qin, Z. Preparation and adsorption performance of functionalization cellulose-based composite aerogel. Int. J. Biol. Macromol. 2022, 211, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Z.; Yang, S.; Zeng, Z.; Zhang, L. Different rheological behaviours of cellulose/tetrabutylammonium acetate/dimethyl sulfoxide/water mixtures. Cellulose 2020, 27, 7967–7978. [Google Scholar] [CrossRef]
- Zeng, Z.; Yang, S.; Zhang, L.; Tang, R.; Zhang, L. One-pot synthesis of cellulose/silver nanoparticle fibers and their antibacterial application. Bioresources 2021, 16, 3360–3376. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, K.; Liu, W.; Tang, W.; Yong, Q. Tuning the cellulose nanocrystal alignments for supramolecular assembly of chiral nematic films with highly efficient UVB shielding capability. J. Mater. Chem. C 2020, 8, 8493–8501. [Google Scholar] [CrossRef]
- Tu, H.; Zhu, M.; Duan, B.; Zhang, L. Recent Progress in High-Strength and Robust Regenerated Cellulose Materials. Adv. Mater. 2020, 33, 2000682. [Google Scholar] [CrossRef]
- Fu, F.; Gu, J.; Cao, J.; Shen, R.; Liu, H.; Zhang, Y.; Liu, X.D.; Zhou, J. Reduction of Silver Ions Using an Alkaline Cellulose Dope: Straightforward Access to Ag/ZnO Decorated Cellulose Nanocomposite Film with Enhanced Antibacterial Activities. ACS Sustain. Chem. Eng. 2017, 6, 738–748. [Google Scholar] [CrossRef]
- Xiong, W.; Li, L.; Qiao, F.; Chen, J.; Xie, Y. Air Superhydrophilic-Superoleophobic SiO2-Based Coatings for Recoverable Oil/Water Separation Mesh with High Flux and Mechanical Stability. J. Colloid Interface Sci. 2021, 600, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Deng, X.; Dong, Y.; Zhou, Z.; Zhang, Y.; Yu, J.; Cai, J.; Zhang, Y. High-strength, transparent and superhydrophobic nanocellulose/nanochitin membranes fabricated via crosslinking of nanofibers and coating F-SiO2 suspensions. Carbohyd. Polym. 2020, 247, 116694. [Google Scholar] [CrossRef]
- Reddy, J.P.; Rajulu, A.V.; Rhim, J.; Seo, J. Mechanical, thermal, and water vapor barrier properties of regenerated cellulose/nano-SiO2 composite films. Cellulose 2018, 25, 7153–7165. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Cha, R.; Long, K.; Li, J.; Jiang, X. Manufacture of Hydrophobic Nanocomposite Films with High Printability. ACS Sustain. Chem. Eng. 2019, 7, 15404–15412. [Google Scholar] [CrossRef]
- Deng, S.; Huang, R.; Zhou, M.; Chen, F.; Fu, Q. Hydrophobic cellulose films with excellent strength and toughness via ball milling activated acylation of microfibrillated cellulose. Carbohyd. Polym. 2016, 154, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Luo, Z.; Wang, C.; Hao, X.; Gao, J. Preparation and characterization of bionanocomposite fiber based on cellulose and nano-SiO2 using ionic liquid. Carbohyd. Polym. 2013, 98, 161–167. [Google Scholar] [CrossRef]
- Rukmanikrishnan, B.; Ramalingam, S.; Kim, S.S.; Lee, J. Rheological and anti-microbial study of silica and silver nanoparticles-reinforced k-carrageenan/hydroxyethyl cellulose composites for food packaging applications. Cellulose 2021, 28, 5577–5590. [Google Scholar] [CrossRef]
- Wang, B.; Lou, W.; Wang, X.; Hao, J. Relationship between dispersion state and reinforcement effect of graphene oxide in microcrystalline cellulose–graphene oxide composite films. J. Mater. Chem. 2012, 22, 12859–12866. [Google Scholar] [CrossRef]
- Ashori, A.; Sheykhnazari, S.; Tabarsa, T.; Shakeri, A.; Golalipour, M. Bacterial cellulose/silica nanocomposites: Preparation and characterization. Carbohyd. Polym. 2012, 90, 413–418. [Google Scholar] [CrossRef]
- Kadokawa, J.I.; Murakami, M.A.; Takegawa, A.; Kaneko, Y. Preparation of cellulose–starch composite gel and fibrous material from a mixture of the polysaccharides in ionic liquid. Carbohyd. Polym. 2009, 75, 180–183. [Google Scholar] [CrossRef]
- Li, R.; Chang, C.; Zhou, J.; Zhang, L.; Gu, W.; Li, C.; Liu, S.; Kuga, S. Primarily Industrialized Trial of Novel Fibers Spun from Cellulose Dope in NaOH/Urea Aqueous Solution. Ind. Eng. Chem. Res. 2010, 49, 11380–11384. [Google Scholar] [CrossRef]
- Zhang, J.; Tominaga, K.; Yamagishi, N.; Gotoh, Y. Comparison of Regenerated Cellulose Fibers Spun from Ionic Liquid Solutions with Lyocell Fiber. J. Fiber Sci. Technol. 2020, 76, 257–266. [Google Scholar] [CrossRef]
- James, W.; Mendon, S.K.; Shelby, F.; Ford, E. X-ray Diffraction of Cotton Treated with Neutralized Vegetable Oil-based Macromolecular Crosslinkers. J. Eng. Fibers Fabrics 2010, 5, 10–20. [Google Scholar]
- Ye, D.; Lei, X.; Li, T.; Cheng, Q.; Chang, C.; Hu, L.; Zhang, L. Ultrahigh Tough, Super Clear, and Highly Anisotropic Nanofiber-Structured Regenerated Cellulose Films. ACS Nano 2019, 13, 4843–4853. [Google Scholar] [CrossRef]
- Miao, J.; Yu, Y.; Jiang, Z.; Tang, L.; Zhang, L. Partial delignification of wood and membrane preparation using a quaternary ammonium ionic liquid. Sci. Rep. 2017, 7, 42472. [Google Scholar] [CrossRef] [Green Version]
- Kamide, K.; Okajima, K.; Kowsaka, K. Dissolution of Natural Cellulose into Aqueous Alkali Solution: Role of Super-Molecular Structure of Cellulose. Polym. J. 1992, 24, 71–86. [Google Scholar] [CrossRef]
- Song, H.; Zheng, L. Nanocomposite films based on cellulose reinforced with nano-SiO2: Microstructure, hydrophilicity, thermal stability, and mechanical properties. Cellulose 2013, 20, 1737–1746. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q. Regenerated cellulose/multiwalled carbon nanotube composite films with enhanced mechanical properties prepared in NaOH/urea aqueous solution. Indian J. Fibre Text. 2017, 42, 51–56. [Google Scholar]
- Shang, X.Y.; Zhu, Z.K.; Yin, J.; Ma, X.D. Compatibility of Soluble Polyimide/Silica Hybrids Induced by a Coupling Agent. Chem. Mater. 2002, 14, 71–77. [Google Scholar] [CrossRef]
- Kim, S.H.; Ahn, S.H.; Hirai, T. Crystallization kinetics and nucleation activity of silica nanoparticle-filled poly(ethylene 2,6-naphthalate). Polymer 2003, 44, 5625–5634. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, M.Q.; Rong, M.Z.; Friedrich, K. Silica nanoparticles filled polypropylene: Effects of particle surface treatment, matrix ductility and particle species on mechanical performance of the composites. Compos. Sci. Technol. 2005, 65, 635–645. [Google Scholar] [CrossRef]
- Zyl, W.; García, M.; Schrauwen, B.; Kooi, B.J.; Verweij, H. Hybrid Polyamide/Silica Nanocomposites: Synthesis and Mechanical Testing. Macromol. Mater. Eng. 2015, 287, 106–110. [Google Scholar]
- Qi, H.; Liu, J.; Gao, S.; Mder, E. Multifunctional films composed of carbon nanotubes and cellulose regenerated from alkaline–urea solution. J. Mater. Chem. A 2013, 1, 2161–2168. [Google Scholar] [CrossRef]
- Han, D.; Yan, L.; Chen, W.; Wan, L.; Bangal, P.R. Cellulose/graphite oxide composite films with improved mechanical properties over a wide range of temperature. Carbohyd. Polym. 2011, 83, 966–972. [Google Scholar] [CrossRef]
- Yang, Q.; Fukuzumi, H.; Saito, T.; Isogai, A.; Zhang, L. Transparent Cellulose Films with High Gas Barrier Properties Fabricated from Aqueous Alkali/Urea Solutions. Biomacromolecules 2011, 12, 2766–2771. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Y.; Feng, J.; Wu, P. Graphene-Oxide-Sheet-Induced Gelation of Cellulose and Promoted Mechanical Properties of Composite Aerogels. J. Phys. Chem. C 2012, 116, 8063–8068. [Google Scholar] [CrossRef]
- Joung, Y.S.; Buie, C.R. Electrophoretic deposition of unstable colloidal suspensions for superhydrophobic surfaces. Langmuir 2011, 27, 4156–4163. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, W.; Hao, D.; Li, L.; Liu, H.; Lu, Z. Functional nanostructured surfaces in hybrid sol–gel glass in large area for antireflective and super-hydrophobic purposes. J. Mater. Chem. 2012, 22, 17328–17331. [Google Scholar] [CrossRef]
- Portugal, I.S.; Dias, V.N.M.; Duarte, R.F.; Evtuguin, D.V. Hydration of cellulose/silica hybrids assessed by sorption isotherms. J. Phys. Chem. B 2010, 114, 4047. [Google Scholar] [CrossRef] [PubMed]
- Chirkova, J.; Andersons, B.; Andersone, I. Determination of standard isotherms of the sorption of some vapors with cellulose. J. Colloid Interface Sci. 2004, 276, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Hult, E.L.; Larsson, P.T.; Iversen, T. Cellulose fibril aggregation—An inherent property of kraft pulps. Polymer 2001, 42, 3309–3314. [Google Scholar] [CrossRef]
- Cai, J.; Liu, S.; Feng, J.; Kimura, S.; Wada, M. Cellulose–Silica Nanocomposite Aerogels by In Situ Formation of Silica in Cellulose Gel. Angew. Chem. Int. Ed. 2011, 51, 2076–2079. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-Hydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Liu, Y.; Moevius, L.; Xu, X.; Qian, T.; Yeomans, J.M.; Wang, Z. Pancake bouncing on superhydrophobic surfaces. Nat. Phys. 2014, 10, 515–519. [Google Scholar] [CrossRef] [Green Version]
- Qasim, U.; Osman, A.I.; Al-Muhtaseb, A.A.H.; Farrell, C.; Al-Abri, M.; Ali, M.; Vo, D.N.; Jamil, F.; Rooney, D.W. Renewable cellulosic nanocomposites for food packaging to avoid fossil fuel plastic pollution: A review. Environ. Chem. Lett. 2020, 19, 613–641. [Google Scholar] [CrossRef]
- Zhu, K.; Tu, H.; Yang, P.C.; Qiu, C.; Zhang, D.; Lu, A.; Luo, L.; Chen, F.; Liu, X.; Chen, L.; et al. Mechanically Strong Chitin Fibers with Nanofibril Structure, Biocompatibility, and Biodegradability. Chem. Mater. 2019, 6, 2078–2087. [Google Scholar]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Wang, T.; Dai, Y.; Wang, Y.; Ding, Y.; Zhang, L. Surface Engineering of Regenerated Cellulose Nanocomposite Films with High Strength, Ultraviolet Resistance, and a Hydrophobic Surface. Polymers 2023, 15, 1427. https://doi.org/10.3390/polym15061427
Zhu Y, Wang T, Dai Y, Wang Y, Ding Y, Zhang L. Surface Engineering of Regenerated Cellulose Nanocomposite Films with High Strength, Ultraviolet Resistance, and a Hydrophobic Surface. Polymers. 2023; 15(6):1427. https://doi.org/10.3390/polym15061427
Chicago/Turabian StyleZhu, Ying, Tianhao Wang, Yanan Dai, Ye Wang, Yukun Ding, and Liping Zhang. 2023. "Surface Engineering of Regenerated Cellulose Nanocomposite Films with High Strength, Ultraviolet Resistance, and a Hydrophobic Surface" Polymers 15, no. 6: 1427. https://doi.org/10.3390/polym15061427