Argon Plasma Jet-Treated Poly (Vinyl Alcohol)/Chitosan and PEG 400 Plus Mangifera indica Leaf Extract for Electrospun Nanofiber Membranes: In Vitro Study
Abstract
:1. Introduction
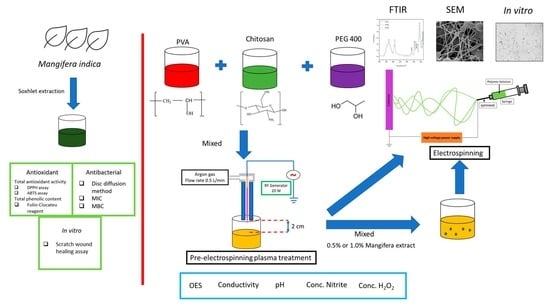
2. Materials and Methods
2.1. Polymer Solution
2.1.1. Material
2.1.2. Preparation of Polymer Solution
2.1.3. Plasma Treatment
2.1.4. Characterization of Prepared Solutions
2.2. Mangifera Extraction (ME)
2.2.1. Sample Preparation
2.2.2. Antioxidant Activity [14,19]
2.2.3. Antimicrobial Activity [14,22]
Disc Diffusion Method (DDM); Zone of Inhibition
Determination of Minimum Inhibitory Concentration (MIC)
Determination of Minimal Bactericidal Concentration (MBC)
2.2.4. In Vitro Cytotoxic Study
Cell Culture of Human Dermal Fibroblast Adult (HDFa)
Scratch wound healing assay
2.3. Electrospun Nanofiber Membrane
2.3.1. Electrospinning Process
2.3.2. Characterization of Nanofiber Membranes
2.3.3. Antibacterial Test
2.3.4. Scratch Wound Healing Assay [31]
2.3.5. Statistical Analysis
3. Results and Discussion
3.1. Plasma-Treated Polymer Solution
3.2. Mangifera Indica Extraction
3.3. Electrospun Nanofiber Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havstad, M.R. Biodegradable plastics. In Plastic Waste and Recycling; Elsevier: Amsterdam, The Netherlands, 2020; pp. 97–129. [Google Scholar]
- Shi, Q.; Vitchuli, N.; Nowak, J.; Lin, Z.; Guo, B.; McCord, M.; Bourham, M.; Zhang, X. Atmospheric plasma treatment of pre-electrospinning polymer solution: A feasible method to improve electrospinnability. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 115–122. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef]
- Sajeev, U.S.; Anoop Anand, K.; Menon, D.; Nair, S. Control of nanostructures in PVA, PVA/chitosan blends and PCL through electrospinning. Bull. Mater. Sci. 2008, 31, 343–351. [Google Scholar] [CrossRef]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun nanofibers: New generation materials for advanced applications. Mater. Sci. Eng. B 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Miguel, S.P.; Sequeira, R.S.; Moreira, A.F.; Cabral, C.S.D.; Mendonca, A.G.; Ferreira, P.; Correia, I.J. An overview of electrospun membranes loaded with bioactive molecules for improving the wound healing process. Eur. J. Pharm. Biopharm. 2019, 139, 1–22. [Google Scholar] [CrossRef]
- Li, M.; Qiu, W.; Wang, Q.; Li, N.; Liu, L.; Wang, X.; Yu, J.; Li, X.; Li, F.; Wu, D. Nitric Oxide-Releasing Tryptophan-Based Poly(ester urea)s Electrospun Composite Nanofiber Mats with Antibacterial and Antibiofilm Activities for Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 15911–15926. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, C.; Wang, Q.; Zhou, F.; Li, T.; Wang, B.; Su, W.; Shang, D.; Wu, S. A simple, quick, and cost-effective strategy to fabricate polycaprolactone/silk fibroin nanofiber yarns for biotextile-based tissue scaffold application. Eur. Polym. J. 2023, 186, 111863. [Google Scholar] [CrossRef]
- Gorji, M.; Sadeghianmaryan, A.; Rajabinejad, H.; Nasherolahkam, S.; Chen, X. Development of Highly pH-Sensitive Hybrid Membranes by Simultaneous Electrospinning of Amphiphilic Nanofibers Reinforced with Graphene Oxide. J. Funct. Biomater. 2019, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, A.G.; Kinandana, A.W.; Sumariyah, S.; Nur, M.; Nandiyanto, A.B.D. Analysis of Plasma-activated Medium (PAM) in aqueous solution by an Atmospheric Pressure Plasma Jet (APPJ). MATEC Web Conf. 2018, 197, 02013. [Google Scholar] [CrossRef] [Green Version]
- Grande, S.; Van Guyse, J.; Nikiforov, A.Y.; Onyshchenko, I.; Asadian, M.; Morent, R.; Hoogenboom, R.; De Geyter, N. Atmospheric pressure plasma jet treatment of poly-ε-caprolactone polymer solutions to improve electrospinning. ACS Appl. Mater. Interfaces 2017, 9, 33080–33090. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, F.; Planckaert, T.; Van Der Voort, P.; Morent, R.; De Geyter, N. Chemical and morphological characterization of nanofibers produced by plasma-treated electrospinning solutions. In Proceedings of the 23rd International Symposium on Plasma Chemistry (ISPC 23), Montréal, QC, Canada, 30 July–4 August 2017; pp. 1006–1009. [Google Scholar]
- Olival, K.J.; Islam, A.; Kabir, M.H.B.; Mannan, M.A.; Islam, M.R. Analgesic, anti-inflammatory and antimicrobial effects of ethanol extracts of mango leaves. J. Bangladesh Agric. Univ. 1970, 8, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Moon, J.Y.; Kim, H.; Lee, D.-S.; Cho, M.; Choi, H.-K.; Kim, Y.S.; Mosaddik, A.; Cho, S.K. Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem. 2010, 121, 429–436. [Google Scholar] [CrossRef]
- Makare, N.; Bodhankar, S.; Rangari, V. Immunomodulatory activity of alcoholic extract of Mangifera indica L. in mice. J. Ethnopharmacol. 2001, 78, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Espinosa, L.; Garduno-Siciliano, L.; Rodriguez-Canales, M.; Hernandez-Portilla, L.B.; Canales-Martinez, M.M.; Rodriguez-Monroy, M.A. The Wound-Healing Effect of Mango Peel Extract on Incision Wounds in a Murine Model. Molecules 2022, 27, 259. [Google Scholar] [CrossRef]
- Nupangtha, W.; Ngamjarurojana, A.; Nisoa, M.; Boonyawan, D. Hydroxyl Density Measurement by Two-Beam UV-LED Absorption Spectroscopy in an Atmospheric-Pressure Ar-H2O2 Vapor HF Plasma Jet. Chiang Mai J. Sci. 2019, 46, 1015–1022. [Google Scholar]
- Mohan, C.G.; Deepak, M.; Viswanatha, G.L.; Savinay, G.; Hanumantharaju, V.; Rajendra, C.E.; Halemani, P.D. Anti-oxidant and anti-inflammatory activity of leaf extracts and fractions of Mangifera indica. Asian Pac. J. Trop. Med. 2013, 6, 311–314. [Google Scholar] [CrossRef] [Green Version]
- Mokbul, M.; Cheow, Y.L.; Siow, L.F. Characterization of the physicochemical properties of mango (Mangifera indica L., Dragon variety) kernel fat extracted via supercritical carbon dioxide and Soxhlet techniques. J. Food Process. Preserv. 2022, 46, e16799. [Google Scholar] [CrossRef]
- Chaovanalikit, A.; Wrolstad, R.E. Total Anthocyanins and Total Phenolics of Fresh and Processed Cherries and Their Antioxidant Properties. J. Food Sci. 2004, 69, FCT67–FCT72. [Google Scholar] [CrossRef]
- Bbosa, G.S.; Kyegombe, D.B.; Ogwal-Okeng, J.; Bukenya-Ziraba, R.; Odyek, O.; Waako, P. Antibacterial activity of Mangifera indica (L.). Afr. J. Ecol. 2007, 45, 13–16. [Google Scholar] [CrossRef]
- Rezaei, F.; Nikiforov, A.; Morent, R.; De Geyter, N. Plasma Modification of Poly Lactic Acid Solutions to Generate High Quality Electrospun PLA Nanofibers. Sci. Rep. 2018, 8, 2241. [Google Scholar] [CrossRef] [Green Version]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbelaez, P.; Cruz, J.C.; Munoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef] [PubMed]
- Sriyai, M.; Tasati, J.; Molloy, R.; Meepowpan, P.; Somsunan, R.; Worajittiphon, P.; Daranarong, D.; Meerak, J.; Punyodom, W. Development of an Antimicrobial-Coated Absorbable Monofilament Suture from a Medical-Grade Poly(l-lactide-co-epsilon-caprolactone) Copolymer. ACS Omega 2021, 6, 28788–28803. [Google Scholar] [CrossRef] [PubMed]
- Daranarong, D.; Chan, R.T.; Wanandy, N.S.; Molloy, R.; Punyodom, W.; Foster, L.J. Electrospun polyhydroxybutyrate and poly(L-lactide-co-epsilon-caprolactone) composites as nanofibrous scaffolds. Biomed. Res. Int. 2014, 2014, 741408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatsee, S.; Daranarong, D.; Punyodom, W.; Worajittiphon, P. Electrospinning polymer blend of PLA and PBAT: Electrospinnability-solubility map and effect of polymer solution parameters toward application as antibiotic-carrier mats. J. Appl. Polym. Sci. 2018, 135, 46486. [Google Scholar] [CrossRef]
- Techaikool, P.; Daranarong, D.; Kongsuk, J.; Boonyawan, D.; Haron, N.; Harley, W.S.; Thomson, K.A.; Foster, L.J.R.; Punyodom, W. Effects of plasma treatment on biocompatibility of poly[(L-lactide)-co-(ϵ-caprolactone)] and poly[(L-lactide)-co-glycolide] electrospun nanofibrous membranes. Polym. Int. 2017, 66, 1640–1650. [Google Scholar] [CrossRef]
- Kudryavtseva, V.; Stankevich, K.; Gudima, A.; Kibler, E.; Zhukov, Y.; Bolbasov, E.; Malashicheva, A.; Zhuravlev, M.; Riabov, V.; Liu, T.; et al. Atmospheric pressure plasma assisted immobilization of hyaluronic acid on tissue engineering PLA-based scaffolds and its effect on primary human macrophages. Mater. Des. 2017, 127, 261–271. [Google Scholar] [CrossRef]
- Rezaei, F.; Shokri, B.; Sharifian, M. Atmospheric-pressure DBD plasma-assisted surface modification of polymethyl methacrylate: A study on cell growth/proliferation and antibacterial properties. Appl. Surf. Sci. 2016, 360, 641–651. [Google Scholar] [CrossRef]
- Arik, N.; Inan, A.; Ibis, F.; Demirci, E.A.; Karaman, O.; Ercan, U.K.; Horzum, N. Modification of electrospun PVA/PAA scaffolds by cold atmospheric plasma: Alignment, antibacterial activity, and biocompatibility. Polym. Bull. 2018, 76, 797–812. [Google Scholar] [CrossRef]
- Rathore, V.; Nema, S.K. Optimization of process parameters to generate plasma activated water and study of physicochemical properties of plasma activated solutions at optimum condition. J. Appl. Phys. 2021, 129, 084901. [Google Scholar] [CrossRef]
- Pang, B.; Liu, Z.; Zhang, H.; Wang, S.; Gao, Y.; Xu, D.; Liu, D.; Kong, M.G. Investigation of the chemical characteristics and anticancer effect of plasma-activated water: The effect of liquid temperature. Plasma Process. Polym. 2021, 19, 2100079. [Google Scholar] [CrossRef]
- Lai, C.C.; Deng, Y.X.; Liao, Y.H. A study on the influence of gas mixtures on the property of plasma-activated water. Plasma Process. Polym. 2019, 17, 1900196. [Google Scholar] [CrossRef]
- Wang, T.; Turhan, M.; Gunasekaran, S. Selected properties of pH-sensitive, biodegradable chitosan–poly(vinyl alcohol) hydrogel. Polym. Int. 2004, 53, 911–918. [Google Scholar] [CrossRef]
- Islam, A.; Yasin, T. Controlled delivery of drug from pH sensitive chitosan/poly (vinyl alcohol) blend. Carbohydr. Polym. 2012, 88, 1055–1060. [Google Scholar] [CrossRef]
- Keun Son, W.; Ho Youk, J.; Seung Lee, T.; Park, W.H. Effect of pH on electrospinning of poly(vinyl alcohol). Mater. Lett. 2005, 59, 1571–1575. [Google Scholar] [CrossRef]
- Li, T.-T.; Yan, M.; Zhong, Y.; Ren, H.-T.; Lou, C.-W.; Huang, S.-Y.; Lin, J.-H. Processing and characterizations of rotary linear needleless electrospun polyvinyl alcohol (PVA)/Chitosan (CS)/Graphene (Gr) nanofibrous membranes. J. Mater. Res. Technol. 2019, 8, 5124–5132. [Google Scholar] [CrossRef]
- Chingsungnoen, A.; Wilson, J.; Amornkitbamrung, V.; Thomas, C.; Burinprakhon, T. Spatially resolved atomic excitation temperatures in CH4/H2 and C3H8/H2 RF discharges by optical emission spectroscopy. Plasma Sources Sci. Technol. 2007, 16, 434. [Google Scholar] [CrossRef]
- Sarani, A.; Nikiforov, A.Y.; Leys, C. Atmospheric pressure plasma jet in Ar and Ar/H2O mixtures: Optical emission spectroscopy and temperature measurements. Phys. Plasmas 2010, 17, 063504. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; He, B.; Chen, Q.; Li, J.; Xiong, Q.; Yue, G.; Zhang, X.; Yang, S.; Liu, H.; Liu, Q.H. Direct synthesis of hydrogen peroxide from plasma-water interactions. Sci. Rep. 2016, 6, 38454. [Google Scholar] [CrossRef]
- Schmitz, E.; Nordberg Karlsson, E.; Adlercreutz, P. Warming weather changes the chemical composition of oat hulls. Plant Biol. 2020, 22, 1086–1091. [Google Scholar] [CrossRef]
- Gupta, S.R.; Singh, J.S. The effect of plant species, weather variables and chemical composition of plant material on decomposition in a tropical grassland. Plant Soil 1981, 59, 99–117. [Google Scholar] [CrossRef]
- Bilgen, F.; Ural, A.; Kurutas, E.B.; Bekerecioglu, M. The effect of oxidative stress and Raftlin levels on wound healing. Int. Wound J. 2019, 16, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Tumpa, S.I.; Hossain, M.I.; Ishika, T. Antimicrobial activities of Psidium guajava, Carica papaya and Mangifera indica against some gram positive and gram negative bacteria. J. Pharmacogn. Phytochem. 2015, 3, 125–129. [Google Scholar]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [Green Version]
- Allaw, M.; Pleguezuelos-Villa, M.; Manca, M.L.; Caddeo, C.; Aroffu, M.; Nacher, A.; Diez-Sales, O.; Sauri, A.R.; Ferrer, E.E.; Fadda, A.M.; et al. Innovative strategies to treat skin wounds with mangiferin: Fabrication of transfersomes modified with glycols and mucin. Nanomedicine 2020, 15, 1671–1685. [Google Scholar] [CrossRef]
- Korting, H.C.; Schollmann, C.; White, R.J. Management of minor acute cutaneous wounds: Importance of wound healing in a moist environment. J. Eur. Acad. Derm. Venereol. 2011, 25, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Gantwerker, E.A.; Hom, D.B. Skin: Histology and physiology of wound healing. Clin. Plast. Surg. 2012, 39, 85–97. [Google Scholar] [CrossRef]
- Johnson, T.R.; Gomez, B.I.; McIntyre, M.K.; Dubick, M.A.; Christy, R.J.; Nicholson, S.E.; Burmeister, D.M. The Cutaneous Microbiome and Wounds: New Molecular Targets to Promote Wound Healing. Int. J. Mol. Sci. 2018, 19, 2699. [Google Scholar] [CrossRef] [Green Version]
- Barakat, S.; Nasr, M.; Ahmed, R.F.; Badawy, S.; Mortada, N. Recent Formulation Advances of Mangiferin. Rev. Bras. De Farmacogn. 2022, 32, 871–882. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Beck Tan, N.C. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Tan, S.H.; Inai, R.; Kotaki, M.; Ramakrishna, S. Systematic parameter study for ultra-fine fiber fabrication via electrospinning process. Polymer 2005, 46, 6128–6134. [Google Scholar] [CrossRef]
- Rwei, S.-P.; Huang, C.-C. Electrospinning PVA solution-rheology and morphology analyses. Fibers Polym. 2012, 13, 44–50. [Google Scholar] [CrossRef]
- Angammana, C.J.; Jayaram, S.H. Analysis of the Effects of Solution Conductivity on Electrospinning Process and Fiber Morphology. IEEE Trans. Ind. Appl. 2011, 47, 1109–1117. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Klingner, A. A review on electrospun polymeric nanofibers: Production parameters and potential applications. Polym. Test. 2020, 90, 106647. [Google Scholar] [CrossRef]
- Valizadeh, A.; Mussa Farkhani, S. Electrospinning and electrospun nanofibres. IET Nanobiotechnol. 2014, 8, 83–92. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, X.; Wu, L.; Han, Y.; Sheng, J. Study on morphology of electrospun poly (vinyl alcohol) mats. Eur. Polym. J. 2005, 41, 423–432. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; Menazea, A.A.; Ismail, A.M. Synthesis, characterization and antimicrobial activity of Chitosan/Polyvinyl Alcohol blend doped with Hibiscus Sabdariffa L. extract. J. Mol. Struct. 2019, 1197, 603–609. [Google Scholar] [CrossRef]
- Anicuta, S.-G.; Dobre, L.; Stroescu, M.; Jipa, I. Fourier transform infrared (FTIR) spectroscopy for characterization of antimicrobial films containing chitosan. An. Univ. Ńii Din Oradea Fasc. Ecotoxicologie Zooteh. Şi Tehnol. De Ind. Aliment. 2010, 2010, 1234–1240. [Google Scholar]
- Alhosseini, S.N.; Moztarzadeh, F.; Mozafari, M.; Asgari, S.; Dodel, M.; Samadikuchaksaraei, A.; Kargozar, S.; Jalali, N. Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. Int. J. Nanomed. 2012, 7, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Fathi, A.; Khanmohammadi, M.; Goodarzi, A.; Foroutani, L.; Mobarakeh, Z.T.; Saremi, J.; Arabpour, Z.; Ai, J. Fabrication of chitosan-polyvinyl alcohol and silk electrospun fiber seeded with differentiated keratinocyte for skin tissue regeneration in animal wound model. J. Biol. Eng. 2020, 14, 27. [Google Scholar] [CrossRef]
- Feizollahi, E.; Arshad, M.; Yadav, B.; Ullah, A.; Roopesh, M.S. Degradation of Deoxynivalenol by Atmospheric-Pressure Cold Plasma and Sequential Treatments with Heat and UV Light. Food Eng. Rev. 2020, 13, 696–705. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grondahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-T.; Gong, J.; Gu, X.-H.; Kim, H.-Y.; Dong, J.; Shen, X.-Y. Fabrication and characterization of poly (vinyl alcohol)/chitosan blend nanofibers produced by electrospinning method. Carbohydr. Polym. 2007, 67, 403–409. [Google Scholar] [CrossRef]
- Naveen Kumar, H.M.P.; Prabhakar, M.N.; Venkata Prasad, C.; Madhusudhan Rao, K.; Ashok Kumar Reddy, T.V.; Chowdoji Rao, K.; Subha, M.C.S. Compatibility studies of chitosan/PVA blend in 2% aqueous acetic acid solution at 30 °C. Carbohydr. Polym. 2010, 82, 251–255. [Google Scholar] [CrossRef]
- Ali, M.; Gherissi, A. Synthesis and characterization of the composite material PVA/chitosan/5% sorbitol with different ratio of chitosan. Int. J. Mech. Mechatron. Eng. 2017, 17, 15–28. [Google Scholar]
- Darbasizadeh, B.; Fatahi, Y.; Feyzi-Barnaji, B.; Arabi, M.; Motasadizadeh, H.; Farhadnejad, H.; Moraffah, F.; Rabiee, N. Crosslinked-polyvinyl alcohol-carboxymethyl cellulose/ZnO nanocomposite fibrous mats containing erythromycin (PVA-CMC/ZnO-EM): Fabrication, characterization and in-vitro release and anti-bacterial properties. Int. J. Biol. Macromol. 2019, 141, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Ojah, N.; Saikia, D.; Gogoi, D.; Baishya, P.; Ahmed, G.A.; Ramteke, A.; Choudhury, A.J. Surface modification of core-shell silk/PVA nanofibers by oxygen dielectric barrier discharge plasma: Studies of physico-chemical properties and drug release behavior. Appl. Surf. Sci. 2019, 475, 219–229. [Google Scholar] [CrossRef]
- Vega-Cázarez, C.A.; López-Cervantes, J.; Sánchez-Machado, D.I.; Madera-Santana, T.J.; Soto-Cota, A.; Ramírez-Wong, B. Preparation and Properties of Chitosan–PVA Fibers Produced by Wet Spinning. J. Polym. Environ. 2017, 26, 946–958. [Google Scholar] [CrossRef]
- Cui, Z.; Zheng, Z.; Lin, L.; Si, J.; Wang, Q.; Peng, X.; Chen, W. Electrospinning and crosslinking of polyvinyl alcohol/chitosan composite nanofiber for transdermal drug delivery. Adv. Polym. Technol. 2018, 37, 1917–1928. [Google Scholar] [CrossRef]
- de Souza, J.R.R.; Feitosa, J.P.A.; Ricardo, N.M.P.S.; Trevisan, M.T.S.; de Paula, H.C.B.; Ulrich, C.M.; Owen, R.W. Spray-drying encapsulation of mangiferin using natural polymers. Food Hydrocoll. 2013, 33, 10–18. [Google Scholar] [CrossRef]
- Wang, J.; Liang, J.; Sun, L.; Gao, S. PVA/CS and PVA/CS/Fe gel beads’ synthesis mechanism and their performance in cultivating anaerobic granular sludge. Chemosphere 2019, 219, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Gwon, J.G.; Lee, S.Y.; Doh, G.H.; Kim, J.H. Characterization of chemically modified wood fibers using FTIR spectroscopy for biocomposites. J. Appl. Polym. Sci. 2010, 116, 3212–3219. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; ur Rehman, D.I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Mansur, H.S.; Oréfice, R.L.; Mansur, A.A.P. Characterization of poly(vinyl alcohol)/poly(ethylene glycol) hydrogels and PVA-derived hybrids by small-angle X-ray scattering and FTIR spectroscopy. Polymer 2004, 45, 7193–7202. [Google Scholar] [CrossRef]
- Achari, D.D.; Hegde, S.N.; Pattanashetti, N.A.; Kamble, R.R.; Kariduraganavar, M.Y. Development of zeolite-A incorporated PVA/CS nanofibrous composite membranes using the electrospinning technique for pervaporation dehydration of water/tert-butanol. New J. Chem. 2021, 45, 3981–3996. [Google Scholar] [CrossRef]
- Agrawal, P.; Pramanik, K. Chitosan-poly(vinyl alcohol) nanofibers by free surface electrospinning for tissue engineering applications. Tissue Eng. Regen. Med. 2016, 13, 485–497. [Google Scholar] [CrossRef]
- Askari, M.; Rezaei, B.; Shoushtari, A.M.; Noorpanah, P.; Abdouss, M.; Ghani, M. Fabrication of high performance chitosan/polyvinyl alcohol nanofibrous mat with controlled morphology and optimised diameter. Can. J. Chem. Eng. 2014, 92, 1008–1015. [Google Scholar] [CrossRef]
- Hansen, E.W.; Holm, K.H.; Jahr, D.M.; Olafsen, K.; Stori, A. Reaction of poly(vinyl alcohol) and dialdehydes during gel formation probed by 1H n.m.r.—A kinetic study. Polymer 1997, 38, 4863–4871. [Google Scholar] [CrossRef]
- Rudra, R.; Kumar, V.; Kundu, P.P. Acid catalysed cross-linking of poly vinyl alcohol (PVA) by glutaraldehyde: Effect of crosslink density on the characteristics of PVA membranes used in single chambered microbial fuel cells. RSC Adv. 2015, 5, 83436–83447. [Google Scholar] [CrossRef]










| Sample | Formulation |
|---|---|
| Control | 4.5%PVA/0.05%CS/PEG 400/ |
| 0.5% Mangifera extract (0.5% ME) | 4.5%PVA0.05%/CS/PEG 400/0.5%Mangifera extract |
| 1.0% Mangifera extract (1.0% ME) | 4.5%PVA0.05%/CS/PEG 400/1.0%Mangifera extract |
| Time of Plasma Treatment (Min) | 0 Min | 30 Min | 60 Min |
|---|---|---|---|
| Conductivity (mS/cm) | 298.33 ± 1.53 | 318.00 ± 4.36 * | 330.00 ± 6.24 * |
| Viscosity (cP or mPa∙s) | 269.40 ± 1.50 | 295.93 ± 2.73 * | 330.68 ± 2.04 * |
| Temperature (°C) | 27.13 ± 0.21 | 27.93 ± 0.15 | 29.07 ± 0.15 |
| pH | 3.92 ± 0.01 | 3.98 ± 0.01 | 4.01 ± 0.01 |
| NO2− (mM) | 5.03 ± 3.94 | 15.79 ± 4.24 * | 22.97 ± 1.60 * |
| NO2− (mg/L) | 3.47 ± 2.72 | 10.89 ± 2.93 * | 15.85 ± 1.01 * |
| H2O2 (mg/L) | 0 | 3.78 ± 0.00 | 5.62 ± 0.01 |
| Sample | Total Antioxidant Activity | Total Phenolic Content | |
|---|---|---|---|
| DPPH Assay IC50 (mg/mL) | ABTS Assay IC50 (mg/mL) | Folin–Ciocalteu Reagent (mg GAE/Extract 1 g) | |
| Sample 1 | 0.93 ± 0.19 | 1.28 ± 0.07 | 30.07 ± 0.04 |
| Sample 2 | 3.79 ± 0.09 * | 4.48 ± 0.83 * | 316.17 ± 18.47 * |
| Sample 3 | 0.68 ± 0.01 | 1.17 ± 0.01 | 39.82 ± 6.71 |
| Sample | Diameter of Inhibition Zone (Mean ± SD) (mm) | |
|---|---|---|
| Staphylococcus aureus (S. aureus) | Escherichia coli (E. coli) | |
| Ethanol | 9.7 ± 0.6 | 9.3 ± 1.2 |
| Mangifera extract | 11.3 ± 1.2 | 9.7 ± 0.6 |
| Ampicillin (4 mg/5 mL) | 34.3 ± 1.2 | 24.7 ± 0.6 |
| Sample | MIC (mg/mL) | MBC (mg/mL) | ||
|---|---|---|---|---|
| S. aureus | E. coli | S. aureus | E. coli | |
| M. indica | 6.25 | 6.25 | 12.5 | 12.5 |
| Ampicillin | 0.8 | 0.8 | 0.8 | 0.8 |
| Assignment | Sample | |||
|---|---|---|---|---|
| PVA + CS | PVA + CS + PEG | 0.5% ME | 1.0% ME | |
| -OH and -NH2 stretching vibration | 3322–3341 | 3100–3500 | 3452–3652 | 3452–3652 |
| C-H stretching vibration | 2904–2870 | 2925–2854 | ||
| C-O (amide I) | 1655–1559 | 1655–1559 | 1655–1559 | 1655–1559 |
| Crosslinking in PVA | 1488–1448 | 1488–1448 | 1488–1448 | 1488–1448 |
| C-H bending vibration | 1248–1247 | |||
| C-O of PVA | 1092–1088 | |||
| Skeletal vibration involving C-O of chitosan | 1029–1025 | 1029–1025 | 1029–1025 | 1029–1025 |
| C-C stretching vibration | 944.7–837.1 | 975–830 | 975–830 | 975–830 |
| Pulsation and some types of pyranose ring deformation | 920–539 | 920–539 | 920–539 | 920–539 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukum, P.; Punyodom, W.; Dangtip, S.; Poramapijitwat, P.; Daranarong, D.; Jenvoraphot, T.; Nisoa, M.; Kuensaen, C.; Boonyawan, D. Argon Plasma Jet-Treated Poly (Vinyl Alcohol)/Chitosan and PEG 400 Plus Mangifera indica Leaf Extract for Electrospun Nanofiber Membranes: In Vitro Study. Polymers 2023, 15, 2559. https://doi.org/10.3390/polym15112559
Sukum P, Punyodom W, Dangtip S, Poramapijitwat P, Daranarong D, Jenvoraphot T, Nisoa M, Kuensaen C, Boonyawan D. Argon Plasma Jet-Treated Poly (Vinyl Alcohol)/Chitosan and PEG 400 Plus Mangifera indica Leaf Extract for Electrospun Nanofiber Membranes: In Vitro Study. Polymers. 2023; 15(11):2559. https://doi.org/10.3390/polym15112559
Chicago/Turabian StyleSukum, Pongphun, Winita Punyodom, Somsak Dangtip, Pipath Poramapijitwat, Donraporn Daranarong, Thannaphat Jenvoraphot, Mudtorlep Nisoa, Chakkrapong Kuensaen, and Dheerawan Boonyawan. 2023. "Argon Plasma Jet-Treated Poly (Vinyl Alcohol)/Chitosan and PEG 400 Plus Mangifera indica Leaf Extract for Electrospun Nanofiber Membranes: In Vitro Study" Polymers 15, no. 11: 2559. https://doi.org/10.3390/polym15112559