Pore Structure Tuning of Poly-EGDMA Biomedical Material by Varying the O-Quinone Photoinitiator
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
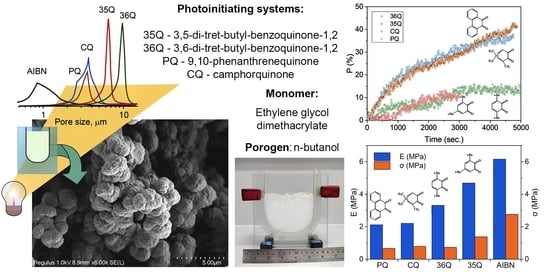
3.1. Porous Structure of Polymers
3.2. Scanning Electron Microscopy
3.3. Mechanical Characteristics of the Polymers
3.4. Kinetics Studies
3.5. Evaluation of the Cytotoxicity of the Polymers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Dedication
References
- Rao, K.M.; Kim, E.; Kim, H.J.; Uthappa, U.T.; Han, S.S. Hyaluronic acid-quercetin pendant drug conjugate for wound healing applications. Int. J. Biol. Macromol. 2023, 240, 124336. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Uthappa, U.; Altalhi, T.; Jung, H.-Y.; Kurkuri, M.D. Functionalized porous hydroxyapatite scaffolds for tissue engineering applications: A focused review. ACS Biomater. Sci. Eng. 2021, 8, 4039–4076. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.S.; Abedini, A.A.; Chen, F.; Whitfield, T.; Ude, C.C.; Laurencin, C.T. Oxygen-Generating Biomaterials for Translational Bone Regenerative Engineering. ACS Appl. Mater. Interfaces, 2023; in press. [Google Scholar] [CrossRef] [PubMed]
- Daneshmandi, L.; Holt, B.D.; Arnold, A.M.; Laurencin, C.T.; Sydlik, S.A. Ultra-low binder content 3D printed calcium phosphate graphene scaffolds as resorbable, osteoinductive matrices that support bone formation in vivo. Sci. Rep. 2022, 12, 6960. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.L.M.; Teo, E.Y.; Chong, M.S.; Liu, Y.; Choolani, M.; Chan, J.K. Advances in bone tissue engineering. In Regenerative Medicine and Tissue Engineering; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Lu, X.; Yu, S.; Chen, G.; Zheng, W.; Peng, J.; Huang, X.; Chen, L. Insight into the roles of melatonin in bone tissue and bone-related diseases. Int. J. Mol. Med. 2021, 47, 82. [Google Scholar] [CrossRef]
- Liu, J.; Curtis, E.; Cooper, C.; Harvey, N.C. State of the art in osteoporosis risk assessment and treatment. J. Endocrinol. Investig. 2019, 42, 1149–1164. [Google Scholar] [CrossRef] [Green Version]
- Jensen, R.K.; Jensen, T.S.; Koes, B.; Hartvigsen, J. Prevalence of lumbar spinal stenosis in general and clinical populations: A systematic review and meta-analysis. Eur. Spine J. 2020, 29, 2143–2163. [Google Scholar] [CrossRef]
- Viswanathan, V.K.; Kanna, R.M. Management of thoracolumbar fractures in adults: Current algorithm. Int. J. Spine 2019, 4, 10–19. [Google Scholar]
- Archunan, M.W.; Petronis, S. Bone Grafts in Trauma and Orthopaedics. Cureus 2021, 13, e17705. [Google Scholar] [CrossRef]
- Bertoldi, S.; Fare, S.; Denegri, M.; Rossi, D.; Haugen, H.; Parolini, O.; Tanzi, M.C. Ability of polyurethane foams to support placenta-derived cell adhesion and osteogenic differentiation: Preliminary results. J. Mater. Sci. Mater. Med. 2010, 21, 1005–1011. [Google Scholar] [CrossRef]
- Kasten, P.; Beyen, I.; Niemeyer, P.; Luginbühl, R.; Bohner, M.; Richter, W. Porosity and pore size of β-tricalcium phosphate scaffold can influence protein production and osteogenic differentiation of human mesenchymal stem cells: An in vitro and in vivo study. Acta Biomater. 2008, 4, 1904–1915. [Google Scholar] [CrossRef] [PubMed]
- Kovylin, R.S.; Aleynik, D.Y.; Fedushkin, I.L. Modern Porous Polymer Implants: Synthesis, Properties, and Application. Polym. Sci. Ser. C 2021, 63, 29–46. [Google Scholar] [CrossRef]
- Chesnokov, S.A.; Lenshina, N.A.; Arsenyev, M.V.; Kovylin, R.S.; Baten’kin, M.A.; Poddel’sky, A.I.; Abakumov, G.A. Preparation of new dioxygen-active triphenylantimony(V) catecholate-containing porous polymer. Appl. Organomet. Chem. 2017, 31, e3553. [Google Scholar] [CrossRef]
- Yudin, V.V.; Kovylin, R.S.; Baten’kin, M.A.; Kulikova, T.I.; Aleynik, D.Y.; Egorikhina, M.N.; Rubtsova, Y.P.; Charykova, I.N.; Mlyavykh, S.G.; Chesnokov, S.A.; et al. Visible-light induced synthesis of biocompatible porous polymers from oligocarbonatedimethacrylate (OCM-2) in the presence of dialkyl phthalates. Polymer 2020, 192, 122302. [Google Scholar] [CrossRef]
- Chesnokov, S.A.; Aleynik, D.Y.; Kovylin, R.S.; Yudin, V.V.; Egiazaryan, T.A.; Egorikhina, M.N.; Zaslavskaya, M.I.; Rubtsova, Y.P.; Gusev, S.A.; Mlyavykh, S.G.; et al. Porous Polymer Scaffolds based on Cross-Linked Poly-EGDMA and PLA: Manufacture, Antibiotics Encapsulation, and In Vitro Study. Macromol. Biosci. 2021, 21, 2000402. [Google Scholar] [CrossRef] [PubMed]
- Bokov, A.; Bulkin, A.; Davydenko, D.; Orlinskaya, N.Y.; Egorikhina, M.; Rubtsova, Y.P.; Charykova, I.; Kovylin, R.; Yudin, V.; Chesnokov, S. Biological Response to a Novel Hybrid Polyoligomer: In vitro and in vivo Models. Sovrem. Tekhnologii V Meditsine 2020, 12, 36–46. [Google Scholar] [CrossRef]
- Kovylin, R.S.; Baten’kin, M.A.; Kulikova, T.I.; Egorikhina, M.N.; Charikova, I.N.; Gusev, S.A.; Rubtsova, Y.P.; Mlyavykh, S.G.; Aleynik, D.Y.; Chesnokov, S.A.; et al. Biocompatible Non-Toxic Porous Polymeric Materials Based on Carbonate- and Phthalate-Containing Dimethacrylates. ChemistrySelect 2019, 4, 4147–4155. [Google Scholar] [CrossRef]
- Kovylin, R.S.; Yudin, V.V.; Shurygina, M.P.; Fedoseev, V.B.; Chesnokov, S.A.; Fedushkin, I.L.; Piskunov, A.V. Porogen Concentration Effect on the Pore Structure and Properties Evolution of Polymer Monolith Based on Oligocarbonate Dimethacrylate OCM-2. Materials 2023, 16, 3177. [Google Scholar] [CrossRef]
- Sinitsyna, E.S.; Vlakh, E.G.; Rober, M.Y.; Tennikova, T.B. Hydrophilic methacrylate monoliths as platforms for protein microarray. Polymer 2011, 52, 2132–2140. [Google Scholar] [CrossRef]
- Xie, S.; Svec, F.; Fréchet, J.M.J. Preparation of porous hydrophilic monoliths: Effect of the polymerization conditions on the porous properties of poly (acrylamide-co-N,N′-methylenebisacrylamide) monolithic rods. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 1013–1021. [Google Scholar] [CrossRef]
- Kovylin, R.; Vlasova, O.; Baten’kin, M.; Kulikova, T.; Chesnokov, S. One-step photolytic synthesis of hydrophobic porous polymer materials by the copolymerization of the dimethacrylate—Alkyl methacrylate system in the presence of methanol. Russ. Chem. Bull. 2019, 68, 1748–1755. [Google Scholar] [CrossRef]
- Shurygina, M.P.; Zakharina, M.Y.; Baten’kin, M.A.; Konev, A.N.; Shavyrin, A.S.; Chelnokov, E.A.; Shushunova, N.Y.; Arsenyev, M.V.; Chesnokov, S.A.; Abakumov, G.A. A blue to red light sensitive photoinitiating systems based on 3,5-di-tert-butyl-o-benzoquinone derivatives for free radical polymerization. Eur. Polym. J. 2020, 127, 109573. [Google Scholar] [CrossRef]
- Kowalska, A.; Sokolowski, J.; Bociong, K. The photoinitiators used in resin based dental composite—A review and future perspectives. Polymers 2021, 13, 470. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, H.; Takahashi, H.; Kanie, T.; Ban, S. Effect of various visible light photoinitiators on the polymerization and color of light-activated resins. Dent. Mater. J. 2009, 28, 454–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalevée, J.; Fouassier, J.-P. Photopolymerisation Initiating Systems; Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Zherebtsov, M.A.; Zhiganshina, E.R.; Lenshina, N.A.; Kovylin, R.S.; Baranov, E.V.; Shushunova, N.Y.; Shurygina, M.P.; Arsenyev, M.V.; Chesnokov, S.A.; Cherkasov, V.K. Synthesis and photoinitiating ability of substituted 4,5-di-tert-alkyl-o-benzoquinones in radical polymerization. Russ. Chem. Bull. 2021, 70, 780–791. [Google Scholar] [CrossRef]
- Ligon, S.C.; Husár, B.; Wutzel, H.; Holman, R.; Liska, R. Strategies to Reduce Oxygen Inhibition in Photoinduced Polymerization. Chem. Rev. 2014, 114, 557–589. [Google Scholar] [CrossRef]
- Shushunova, N.Y.; Chesnokov, S.A. Inhibition of polymerization of methyl methacrylate by an ortho-benzoquinone-amine system. Polym. Sci. Ser. B 2009, 51, 427–437. [Google Scholar] [CrossRef]
- Simandi, T.; Tüdös, F. Kinetics of radical polymerization—XLV. Steric effects in the radical reactivity of quinones. Eur. Polym. J. 1985, 21, 865–869. [Google Scholar] [CrossRef]
- Chesnokov, S.; Chechet, Y.V.; Yudin, V.; Abakumov, G. Photopolymerization of Thick Layers of Compositions for Mask-Based Stereolithographic Synthesis. High Energy Chem. 2019, 53, 413–417. [Google Scholar] [CrossRef]
- Chesnokov, S.A.; Cherkasov, V.K.; Abakumov, G.A.; Mamysheva, O.N.; Zakharina, M.Y.; Shushunova, N.Y.; Chechet, Y.V.; Kuropatov, V.A. Photoinitiation of methacrylate polymerization with an o-benzoquinone-amine system. Polym. Sci. Ser. B 2014, 56, 11–20. [Google Scholar] [CrossRef]
- Chesnokov, S.A.; Cherkasov, V.K.; Abakumov, G.A.; Mamysheva, O.N.; Chechet, Y.V.; Nevodchikov, V.I. Influence of o-benzoquinone nature on initiation of radical polymerization by the o-benzoquinone—Tert-amine system. Russ. Chem. Bull. 2001, 50, 2366–2371. [Google Scholar] [CrossRef]
- Zhiganshina, E.; Arsenyev, M.; Konev, A.; Chechet, Y.; Chesnokov, S. Photopolymerization of OCDMA Dimetacrylate Initiated by 3, 5-Di-tert-Butyl-o-Quinone and its Bis-O-Benzoquinone. In Key Engineering Materials; Trans Tech Publications Ltd.: Baech, Switzerland, 2020; pp. 129–134. [Google Scholar]
- Mensov, S.N.; Abakumov, G.A.; Arsenyev, M.V.; Baten’kin, M.A.; Chesnokov, S.A.; Konev, A.N.; Polushtaytsev, Y.V.; Shurygina, M.P.; Zakharina, M.Y. Use of photodegradable inhibitors in UV-curable compositions to form polymeric 2D-structures by visible light. J. Appl. Polym. Sci. 2020, 137, 48976. [Google Scholar] [CrossRef]
- Jakubiak, J.; Allonas, X.; Fouassier, J.P.; Sionkowska, A.; Andrzejewska, E.; Linden, L.Å.; Rabek, J.F. Camphorquinone–amines photoinitating systems for the initiation of free radical polymerization. Polymer 2003, 44, 5219–5226. [Google Scholar] [CrossRef]
- Asmussen, S.; Arenas, G.; Cook, W.D.; Vallo, C. Photoinitiation rate profiles during polymerization of a dimethacrylate-based resin photoinitiated with camphorquinone/amine. Influence of initiator photobleaching rate. Eur. Polym. J. 2009, 45, 515–522. [Google Scholar] [CrossRef]
- Asmusen, S.; Arenas, G.; Cook, W.D.; Vallo, C. Photobleaching of camphorquinone during polymerization of dimethacrylate-based resins. Dent. Mater. 2009, 25, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Morlet-Savary, F.; Klee, J.E.; Pfefferkorn, F.; Fouassier, J.P.; Lalevée, J. The camphorquinone/amine and camphorquinone/amine/phosphine oxide derivative photoinitiating systems: Overview, mechanistic approach, and role of the excitation light source. Macromol. Chem. Phys. 2015, 216, 2161–2170. [Google Scholar] [CrossRef]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef]
- Dressano, D.; Palialol, A.R.; Xavier, T.A.; Braga, R.R.; Oxman, J.D.; Watts, D.C.; Marchi, G.M.; Lima, A.F. Effect of diphenyliodonium hexafluorophosphate on the physical and chemical properties of ethanolic solvated resins containing camphorquinone and 1-phenyl-1, 2-propanedione sensitizers as initiators. Dent. Mater. 2016, 32, 756–764. [Google Scholar] [CrossRef]
- Maciel, D.d.S.A.; Caires-Filho, A.B.; Fernandez-Garcia, M.; Anauate-Netto, C.; Alonso, R.C.B. Effect of camphorquinone concentration in physical-mechanical properties of experimental flowable resin composites. BioMed Res. Int. 2018, 2018, 7921247. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, P.P.A.; Bertolo, M.L.; Cavalcante, L.M.; Pfeifer, C.; Schneider, L.F. Degree of conversion, depth of cure, and color stability of experimental dental composite formulated with camphorquinone and phenanthrenequinone photoinitiators. J. Esthet. Restor. Dent. 2015, 27, S49–S57. [Google Scholar] [CrossRef]
- Lima, A.F.; Salvador, M.V.O.; Dressano, D.; Saraceni, C.H.C.; Gonçalves, L.S.; Hadis, M.; Palin, W.M. Increased rates of photopolymerisation by ternary type II photoinitiator systems in dental resins. J. Mech. Behav. Biomed. Mater. 2019, 98, 71–78. [Google Scholar] [CrossRef]
- Kirschner, J.; Szillat, F.; Bouzrati-Zerelli, M.; Becht, J.-M.; Klee, J.E.; Lalevée, J. Sulfinates and sulfonates as high performance co-initiators in CQ based systems: Towards aromatic amine-free systems for dental restorative materials. Dent. Mater. 2020, 36, 187–196. [Google Scholar] [CrossRef]
- Len’shina, N.A.; Zakharina, M.Y.; Kovylin, R.S.; Baten’kin, M.A.; Kulikova, T.I.; Arsen’ev, M.V.; Chesnokov, S.A. Photoreduction of 9,10-Phenanthrenequinone in the Presence of Dimethacrylate Oligomers and Their Polymers. High Energy Chem. 2018, 52, 378–383. [Google Scholar] [CrossRef]
- Zakharina, M.Y.; Fedoseev, V.; Chechet, Y.V.; Chesnokov, S.; Shaplov, A. Effect of viscosity of dimethacrylate ester-based compositions on the kinetics of their photopolymerization in presence of o-quinone photoinitiators. Polym. Sci. Ser. B 2017, 59, 665–673. [Google Scholar] [CrossRef]
- Available online: https://www.iso.org/standard/31261.html (accessed on 30 January 2023).
- Chesnokov, S.A.; Cherkasov, V.K.; Checket, Y.V.; Nevodchikov, N.I.; Abakumov, G.A.; Mamysheva, O.N. Photoreduction of ortho-benzoquinones in the presence of para-substituted N,N-dimethylanilines. Russ. Chem. Bull. 2000, 49, 1506–1511. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Shanmugam, S.; Gopal, B. Antimicrobial and cytotoxicity evaluation of aliovalent substituted hydroxyapatite. Appl. Surf. Sci. 2014, 303, 277–281. [Google Scholar] [CrossRef]
- Shalchy, F.; Lovell, C.; Bhaskar, A. Hierarchical porosity in additively manufactured bioengineering scaffolds: Fabrication & characterisation. J. Mech. Behav. Biomed. Mater. 2020, 110, 103968. [Google Scholar]
- Wagoner Johnson, A.J.; Herschler, B.A. A review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repair. Acta Biomater. 2011, 7, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Grijpma, D.W.; Feijen, J. Porous polymeric structures for tissue engineering prepared by a coagulation, compression moulding and salt leaching technique. Biomaterials 2003, 24, 1937–1947. [Google Scholar] [CrossRef]
- Len’shina, N.; Shurygina, M.; Chesnokov, S. Photoreduction Reaction of Carbonyl-Containing Compounds in the Synthesis and Modification of Polymers. Polym. Sci. Ser. B 2021, 63, 657–690. [Google Scholar] [CrossRef]
- Granchak, V.; Chemerskaya, Z.; Dilung, I. Kinetic studies of the photopolymerization of methyl methacrylate in solution by benzophenones in presence of amines. Polym. Sci. USSR 1985, 27, 302–310. [Google Scholar] [CrossRef]
- Chesnokov, S.A.; Shurygina, M.P.; Abakumov, G.A. The mechanism of photoinduced hydrogen transfer during photoreduction of carbonyl compounds. High Energy Chem. 2011, 45, 287–299. [Google Scholar] [CrossRef]
- Nicodem, D.E.; Silva, R.S.; Togashi, D.M.; da Cunha, M.F.V. Solvent effects on the quenching of the equilibrating n, π* and π, π* triplet states of 9, 10-phenanthrenequinone by 2-propanol. J. Photochem. Photobiol. A Chem. 2005, 175, 154–158. [Google Scholar] [CrossRef]
- Patai, S. The Chemistry of the Quinonoid Compounds; Wiley and Sons: London, UK, 1974. [Google Scholar]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef]
- Hadis, M.A.; Shortall, A.C.; Palin, W.M. Competitive light absorbers in photoactive dental resin-based materials. Dent. Mater. 2012, 28, 831–841. [Google Scholar] [CrossRef]
- Shurygina, M.P.; Kurskii, Y.A.; Druzhkov, N.O.; Chesnokov, S.A.; Abakumov, G.A. Products and mechanisms of photochemical transformations of o-quinones. High Energy Chem. 2010, 44, 234–238. [Google Scholar] [CrossRef]
- Shurygina, M.P.; Kurskii, Y.A.; Chesnokov, S.A.; Druzhkov, N.O.; Fukin, G.K.; Abakumov, G.A.; Cherkasov, V.K. o-benzoquinone photoreduction products in the presence of N,N-dimethylanilines. Russ. Chem. Bull. 2006, 55, 1585–1592. [Google Scholar] [CrossRef]
- Zakharina, M.Y.; Chechet, Y.V.; Shurygina, M.; Chesnokov, S. Influence of viscosity of compositions based on dimethacrylate esters on kinetics of their photopolymerization initiated by 9,10-phenanthrenequinone. Polym. Sci. Ser. B 2018, 60, 708–716. [Google Scholar] [CrossRef]
- Shurygina, M.P.; Chesnokov, S.A.; Abakumov, G.A. Effect of donor and acceptor properties of solvents on the kinetics of photoreduction of sterically hindered o-benzoquinones. High Energy Chem. 2016, 50, 356–361. [Google Scholar] [CrossRef]
- Barra, M.; Harder, E.D.; Balfe, J.P. Influence of solvent polarity on the photoreactivity of 2–4-ring aromatic o-quinones. J. Chem. Soc. Perkin Trans. 1999, 2, 1439–1442. [Google Scholar] [CrossRef]
- Silva, R.S.; Nicodem, D.E. Solvent and temperature effects on the phosphorescence of 9,10-phenanthrenequinone in fluid solution. J. Photochem. Photobiol. A Chem. 2004, 162, 231–238. [Google Scholar] [CrossRef]








| Polymer | Dmod, μm | εsk, % | εHg, % | Ssp, m2 g−1 | εsk–εHg, % | E, MPa | σ, MPa |
|---|---|---|---|---|---|---|---|
| 36Q | 9.9 | 77.0 | 75.4 | 43.1 | 1.6 | 3.3 | 0.73 |
| 35Q | 6.4 | 77.6 | 72.2 | 29.5 | 5.4 | 4.7 | 1.38 |
| PQ | 3.6 | 76.0 | 73.5 | 38.4 | 2.5 | 2.1 | 0.68 |
| PQ * | 3.6 | 76.1 | 75.0 | 36.3 | 1.1 | 2.2 | 0.57 |
| CQ | 3.7 | 76.2 | 74.5 | 29.4 | 1.7 | 2.2 | 0.80 |
| AIBN | 0.8 | 77.3 | 76.1 | 60.4 | 1.2 | 6.2 | 2.76 |
| Polymer | Pore Size/Fraction | ||
|---|---|---|---|
| 0.01–1.0 μm, % | 1–12 μm, % | 12–100 μm, % | |
| CQ | 4.60 | 76.90 | 16.58 |
| PQ | 3.05 | 78.54 | 15.86 |
| PQ * | 5.64 | 76.08 | 16.57 |
| 36Q | 3.28 | 85.48 | 9.46 |
| 35Q | 1.46 | 89.21 | 8.54 |
| AIBN | 47.7 | 46.25 | 4.01 |
| Polymer | Illuminance, kLx | Tind, s | Wmax × 104, s−1 | Plim, % |
|---|---|---|---|---|
| PQ | 50 | 80 | 2.7 | 41 |
| CQ | 50 | 140 | 2.2 | 35 |
| 36Q | 50 | 200 | 0.7 | 14 |
| 35Q | 50 | 1000 | 0.7 | 11 |
| PQ * | 10 | 80 | 1.9 | 29 |
| Series | Parameters | Polymer | ||||
|---|---|---|---|---|---|---|
| 36Q | PQ | 35Q | CQ | AIBN | ||
| Control (n = 8) | OD | 0.455 ± 0.08 | 0.524 ± 0.021 | 0.454 ± 0.029 | 0.537 ± 0.014 | 0.553 ± 0.015 |
| RGI, % | 100 | 100 | 100 | 100 | 100 | |
| Cytotoxicity level | 0 | 0 | 0 | 0 | 0 | |
| Extract (n = 8) | OD | 0.559 ± 0.018 | 0.627 ± 0.054 | 0.584 ± 0.022 | 0.679 ± 0.041 | 0.457 ± 0.021 |
| RGI, % | 122 | 119 | 129 | 126 | 83 | |
| Cytotoxicity level | 0 | 0 | 0 | 0 | 1 | |
| Extract 1:1 (n = 8) | OD | 0.496 ± 0.012 | 0.57 ± 0.041 | 0.517 ± 0.017 | 0.615 ± 0.023 | 0.505 ± 0.026 |
| RGI, % | 109 | 108 | 114 | 115 | 91 | |
| Cytotoxicity level | 0 | 0 | 0 | 0 | 1 | |
| Extract 1:2 (n = 8) | OD | 0.485 ± 0.016 | 0.602 ± 0.027 | 0.585 ± 0.013 | 0.674 ± 0.018 | 0.422 ± 0.014 |
| RGI, % | 106 | 115 | 129 | 126 | 76 | |
| Cytotoxicity level | 0 | 0 | 0 | 0 | 1 | |
| Extract 1:4 (n = 8) | OD | 0.507 ± 0.018 | 0.612 ± 0.028 | 0.533 ± 0.027 | 0.722 ± 0.022 | 0.436 ± 0.013 |
| RGI, % | 111 | 117 | 117 | 134 | 79 | |
| Cytotoxicity level | 0 | 0 | 0 | 0 | 1 | |
| Series | Parameters | Polymer | ||||
|---|---|---|---|---|---|---|
| 36Q | PQ | 35Q | CQ | AIBN | ||
| Control (n = 8) | OD | 0.372 ± 0.023 | 0.405 ± 0.021 | 0.417 ± 0.016 | 0.386 ± 0.014 | 0.370 ± 0.013 |
| RGI, % | 100 | 100 | 100 | 100 | 100 | |
| Cytotoxicity level | 0 | 0 | 0 | 0 | 0 | |
| Extract (n = 8) | OD | 0.380 ± 0.01 | 0.494 ± 0.014 | 0.427 ± 0.011 | 0.494 ± 0.023 | 0.429 ± 0.014 |
| RGI, % | 102 | 121 | 102 | 128 | 116 | |
| Cytotoxicity level | 0 | 0 | 0 | 0 | 0 | |
| Extract 1:1 (n = 8) | OD | 0.385 ± 0.009 | 0.506 ± 0.014 | 0.416 ± 0.012 | 0.483 ± 0.024 | 0.467 ± 0.013 |
| RGI, % | 103 | 125 | 99 | 125 | 126 | |
| Cytotoxicity level | 0 | 0 | 1 | 0 | 0 | |
| Extract 1:2 (n = 8) | OD | 0.420 ± 0.013 | 0.481 ± 0.015 | 0.429 ± 0.008 | 0.422 ± 0.010 | 0.439 ± 0.009 |
| RGI, % | 112 | 119 | 103 | 109 | 119 | |
| Cytotoxicity level | 0 | 0 | 0 | 0 | 0 | |
| Extract 1:4 (n = 8) | OD | 0.439 ± 0.013 | 0.417 ± 0.014 | 0.417 ± 0.007 | 0.367 ± 0.023 | 0.475 ± 0.021 |
| RGI, % | 118 | 103 | 100 | 95 | 128 | |
| Cytotoxicity level | 0 | 0 | 0 | 1 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yudin, V.V.; Shurygina, M.P.; Egorikhina, M.N.; Aleynik, D.Y.; Linkova, D.D.; Charykova, I.N.; Kovylin, R.S.; Chesnokov, S.A. Pore Structure Tuning of Poly-EGDMA Biomedical Material by Varying the O-Quinone Photoinitiator. Polymers 2023, 15, 2558. https://doi.org/10.3390/polym15112558
Yudin VV, Shurygina MP, Egorikhina MN, Aleynik DY, Linkova DD, Charykova IN, Kovylin RS, Chesnokov SA. Pore Structure Tuning of Poly-EGDMA Biomedical Material by Varying the O-Quinone Photoinitiator. Polymers. 2023; 15(11):2558. https://doi.org/10.3390/polym15112558
Chicago/Turabian StyleYudin, Vladimir V., Margarita P. Shurygina, Marfa N. Egorikhina, Diana Ya. Aleynik, Daria D. Linkova, Irina N. Charykova, Roman S. Kovylin, and Sergey A. Chesnokov. 2023. "Pore Structure Tuning of Poly-EGDMA Biomedical Material by Varying the O-Quinone Photoinitiator" Polymers 15, no. 11: 2558. https://doi.org/10.3390/polym15112558