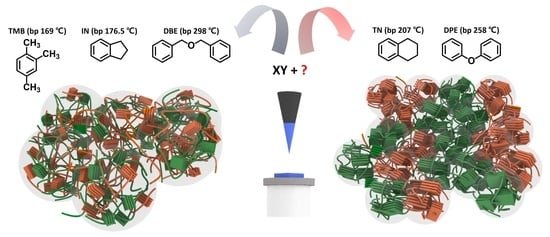
Critical Role of Non-Halogenated Solvent Additives in Eco-Friendly and Efficient All-Polymer Solar Cells
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Xu, G.; Cui, C.; Li, Y. Flexible and Semitransparent Organic Solar Cells. Adv. Energy Mater. 2018, 8, 1701791. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Fang, J.; Li, W.; Shen, Y.; Chen, J.D.; Li, Y.; Gu, H.; Pelivani, S.; Zhang, M.; Li, Y.; et al. Synergetic Transparent Electrode Architecture for Efficient Non-Fullerene Flexible Organic Solar Cells with >12% Efficiency. ACS Nano 2019, 13, 4686–4694. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, J.; Ma, X.; Gao, J.; Xu, C.; Wang, X.; Zhang, X.; Wang, Z.; Zhang, F. Semitransparent organic solar cells exhibiting 13.02% efficiency and 20.2% average visible transmittance. J. Mater. Chem. A 2021, 9, 6797–6804. [Google Scholar] [CrossRef]
- Thompson, B.C.; Frechet, J.M. Polymer-fullerene composite solar cells. Angew. Chem. Int. Ed. 2008, 47, 58–77. [Google Scholar] [CrossRef]
- Clarke, T.M.; Durrant, J.R. Charge Photogeneration in Organic Solar Cells. Chem. Rev. 2010, 110, 6736–6767. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% Efficiency organic solar cells. Sci. Bull. 2020, 65, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, J.; Song, J.; Xu, J.; Zhang, H.; Zhang, X.; Guo, J.; Zhu, L.; Wei, D.; Han, G.; et al. Non-fullerene acceptors with branched side chains and improved molecular packing to exceed 18% efficiency in organic solar cells. Nat. Energy 2021, 6, 605–613. [Google Scholar] [CrossRef]
- Xu, X.; Yu, L.; Meng, H.; Dai, L.; Yan, H.; Li, R.; Peng, Q. Polymer Solar Cells with 18.74% Efficiency: From Bulk Heterojunction to Interdigitated Bulk Heterojunction. Adv. Funct. Mater. 2021, 32, 2108797. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Deng, D.; Zhou, H.; Zhang, J.; Wei, Z. “N-π-N” Type Oligomeric Acceptor Achieves an OPV Efficiency of 18.19% with Low Energy Loss and Excellent Stability. Adv. Sci. 2022, 9, 2202513. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Y.; Xu, Y.; Xian, K.; Bi, P.; Chen, Z.; Zhou, K.; Ma, L.; Zhang, T.; Yang, Y.; et al. A New Polymer Donor Enables Binary All-Polymer Organic Photovoltaic Cells with 18% Efficiency and Excellent Mechanical Robustness. Adv. Mater. 2022, 34, 2205009. [Google Scholar] [CrossRef]
- Sun, R.; Wang, T.; Fan, Q.; Wu, M.; Yang, X.; Wu, X.; Yu, Y.; Xia, X.; Cui, F.; Wan, J.; et al. 18.2%-efficient ternary all-polymer organic solar cells with improved stability enabled by a chlorinated guest polymer acceptor. Joule 2023, 7, 221–237. [Google Scholar] [CrossRef]
- Yang, X.; Sun, R.; Wang, Y.; Chen, M.; Xia, X.; Lu, X.; Lu, G.; Min, J. Ternary All-Polymer Solar Cells with Efficiency up to 18.14% Employing a Two-Step Sequential Deposition. Adv. Mater. 2023, 35, 2209350. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xia, X.; Qiu, B.; Zhang, J.; Qin, S.; Li, X.; Lai, W.; Lu, X.; Meng, L.; Zhang, Z.; et al. Manipulating Polymer Backbone Configuration via Halogenated Asymmetric End-Group Enables Over 18% Efficiency All-Polymer Solar Cells. Adv. Mater. 2023, 2211296. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xie, C.; Li, Q.; Liu, C.; Gao, J.; Jee, M.H.; Qiao, J.; Li, Y.; Song, J.; Hao, X.; et al. Improved Molecular Ordering in a Ternary Blend Enables All-Polymer Solar Cells over 18% Efficiency. Adv. Mater. 2023, 35, 2208165. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, J.; Bi, P.; Ren, J.; Wang, Y.; Yang, Y.; Liu, X.; Zhang, S.; Hou, J. Tandem Organic Solar Cell with 20.2% Efficiency. Joule 2022, 6, 171–184. [Google Scholar] [CrossRef]
- Bi, P.; Wang, J.; Cui, Y.; Zhang, J.; Zhang, T.; Chen, Z.; Qiao, J.; Dai, J.; Zhang, S.; Hao, X.; et al. Enhancing Photon Utilization Efficiency for High-Performance Organic Photovoltaic Cells via Regulating Phase Transition Kinetics. Adv. Mater. 2023, 2210865. [Google Scholar] [CrossRef]
- Genene, Z.; Mammo, W.; Wang, E.; Andersson, M.R. Recent Advances in n-Type Polymers for All-Polymer Solar Cells. Adv. Mater. 2019, 31, 1807275. [Google Scholar] [CrossRef]
- Fan, Q.; An, Q.; Lin, Y.; Xia, Y.; Li, Q.; Zhang, M.; Su, W.; Peng, W.; Zhang, C.; Liu, F.; et al. Over 14% efficiency all-polymer solar cells enabled by a low bandgap polymer acceptor with low energy loss and efficient charge separation. Energy Environ. Sci. 2020, 13, 5017–5027. [Google Scholar] [CrossRef]
- Kim, T.; Younts, R.; Lee, W.; Lee, S.; Gundogdu, K.; Kim, B.J. Impact of the photo-induced degradation of electron acceptors on the photophysics, charge transport and device performance of all-polymer and fullerene–polymer solar cells. J. Mater. Chem. A 2017, 5, 22170–22179. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Ford, M.J.; Li, F.; Sun, J.; Ling, X.; Wang, Y.; Gu, J.; Yuan, J.; Ma, W. Thermally Stable All-Polymer Solar Cells with High Tolerance on Blend Ratios. Adv. Energy Mater. 2018, 8, 1800029. [Google Scholar] [CrossRef]
- Lee, J.-W.; Sun, C.; Ma, B.S.; Kim, H.J.; Wang, C.; Ryu, J.M.; Lim, C.; Kim, T.-S.; Kim, Y.-H.; Kwon, S.-K.; et al. Efficient, Thermally Stable, and Mechanically Robust All-Polymer Solar Cells Consisting of the Same Benzodithiophene Unit-Based Polymer Acceptor and Donor with High Molecular Compatibility. Adv. Energy Mater. 2021, 11, 2003367. [Google Scholar] [CrossRef]
- Du, J.; Hu, K.; Zhang, J.; Meng, L.; Yue, J.; Angunawela, I.; Yan, H.; Qin, S.; Kong, X.; Zhang, Z.; et al. Polymerized small molecular acceptor based all-polymer solar cells with an efficiency of 16.16% via tuning polymer blend morphology by molecular design. Nat. Commun. 2021, 12, 5264. [Google Scholar] [CrossRef]
- Liu, T.; Yang, T.; Ma, R.; Zhan, L.; Luo, Z.; Zhang, G.; Li, Y.; Gao, K.; Xiao, Y.; Yu, J.; et al. 16% efficiency all-polymer organic solar cells enabled by a finely tuned morphology via the design of ternary blend. Joule 2021, 5, 914–930. [Google Scholar] [CrossRef]
- Fan, Q.; Fu, H.; Luo, Z.; Oh, J.; Fan, B.; Lin, F.; Yang, C.; Jen, A.K.-Y. Near-infrared absorbing polymer acceptors enabled by selenophene-fused core and halogenated end-group for binary all-polymer solar cells with efficiency over 16%. Nano Energy 2022, 92, 106718. [Google Scholar] [CrossRef]
- Liao, C.; Gong, Y.; Xu, X.; Yu, L.; Li, R.; Peng, Q. Cost-Efficiency balanced polymer acceptors based on lowly fused Dithienopyrrolo[3, 2b]benzothiadiazole for 16.04% efficiency All-Polymer solar cells. Chem. Eng. J. 2022, 435, 134862. [Google Scholar] [CrossRef]
- Shaheen, S.E.; Brabec, C.J.; Sariciftci, N.S.; Padinger, F.; Fromherz, T.; Hummelen, J.C. 2.5% efficient organic plastic solar cells. Appl. Phys. Lett. 2001, 78, 841–843. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, K.L.; Gu, X.; Kurosawa, T.; Yan, H.; Guo, Y.; Koleilat, G.I.; Zhao, D.; Toney, M.F.; Bao, Z. All-Polymer Solar Cells Employing Non-Halogenated Solvent and Additive. Chem. Mater. 2016, 28, 5037–5042. [Google Scholar] [CrossRef]
- Li, Z.; Ying, L.; Zhu, P.; Zhong, W.; Li, N.; Liu, F.; Huang, F.; Cao, Y. A generic green solvent concept boosting the power conversion efficiency of all-polymer solar cells to 11%. Energy Environ. Sci. 2019, 12, 157–163. [Google Scholar] [CrossRef]
- Coates, N.E.; Hwang, I.-W.; Peet, J.; Bazan, G.C.; Moses, D.; Heeger, A.J. 1,8-octanedithiol as a processing additive for bulk heterojunction materials: Enhanced photoconductive response. Appl. Phys. Lett. 2008, 93, 072105. [Google Scholar] [CrossRef]
- Lee, J.K.; Ma, W.L.; Brabec, C.J.; Yuen, J.; Moon, J.S.; Kim, J.Y.; Lee, K.; Bazan, G.C.; Heeger, A.J. Processing Additives for Improved Efficiency from Bulk Heterojunction Solar Cells. J. Am. Chem. Soc. 2008, 130, 3619–3623. [Google Scholar] [CrossRef]
- Zhao, W.; Ye, L.; Zhang, S.; Sun, M.; Hou, J. A universal halogen-free solvent system for highly efficient polymer solar cells. J. Mater. Chem. A 2015, 3, 12723–12729. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Z.; Zhang, S.; Zheng, Z.; Yang, B.; Ma, W.; Hou, J. Rational selection of solvents and fine tuning of morphologies toward highly efficient polymer solar cells fabricated using green solvents. RSC Adv. 2015, 5, 69567–69572. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Yang, G.; Jiang, K.; Lin, H.; Ade, H.; Ma, W.; Yan, H. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 2016, 1, 15027. [Google Scholar] [CrossRef]
- Jung, H.; Jung, A.-R.; Jin, S.-M.; Kim, S.; Heo, H.; Nguyen, H.V.T.; Kim, M.J.; Ahn, P.; Kim, M.H.; Lee, Y.; et al. Influence of 3D morphology on the performance of all-polymer solar cells processed using environmentally benign nonhalogenated solvents. Nano Energy 2020, 77, 105106. [Google Scholar] [CrossRef]
- Lee, C.; Kang, H.; Lee, W.; Kim, T.; Kim, K.-H.; Woo, H.Y.; Wang, C.; Kim, B.J. High-Performance All-Polymer Solar Cells Via Side-Chain Engineering of the Polymer Acceptor: The Importance of the Polymer Packing Structure and the Nanoscale Blend Morphology. Adv. Mater. 2015, 27, 2466–2471. [Google Scholar] [CrossRef]
- Yang, T.; Yao, S.; Liu, T.; Huang, B.; Xiao, Y.; Liu, H.; Lu, X.; Zou, B. Tailoring the Morphology’s Microevolution for Binary All-Polymer Solar Cells Processed by Aromatic Hydrocarbon Solvent with 16.22% Efficiency. ACS Appl. Mater. Interfaces 2022, 14, 29956–29963. [Google Scholar] [CrossRef]
- Kyaw, A.K.K.; Wang, D.H.; Wynands, D.; Zhang, J.; Nguyen, T.-Q.; Bazan, G.C.; Heeger, A.J. Improved Light Harvesting and Improved Efficiency by Insertion of an Optical Spacer (ZnO) in Solution-Processed Small-Molecule Solar Cells. Nano Lett. 2013, 13, 3796–3801. [Google Scholar] [CrossRef]
- Cowan, S.R.; Leong, W.L.; Banerji, N.; Dennler, G.; Heeger, A.J. Identifying a Threshold Impurity Level for Organic Solar Cells: Enhanced First-Order Recombination Via Well-Defined PC84BM Traps in Organic Bulk Heterojunction Solar Cells. Adv. Funct. Mater. 2011, 21, 3083–3092. [Google Scholar] [CrossRef]
- Choi, J.; Kim, K.; Yu, H.; Lee, C.; Kang, H.; Song, I.; Kim, Y.; Oh, J.H.; Kim, B.J. Importance of Electron Transport Ability in Naphthalene Diimide-Based Polymer Acceptors for High-Performance, Additive-Free, All-Polymer Solar Cells. Chem. Mater. 2015, 27, 5230–5237. [Google Scholar] [CrossRef]













| Solvent | Voc (V) | Jsc (mA/cm2) | FF | PCE (%) a |
|---|---|---|---|---|
| XY | 0.80 ± 0.01 (0.81) | 9.60 ± 0.16 (9.86) | 0.579 ± 0.05 (0.59) | 4.44 ± 0.14 (4.66) |
| XY + TMB | 0.793 ± 0.001 (0.79) | 9.57 ± 0.11 (9.67) | 0.563 ± 0.003 (0.57) | 4.27 ± 0.06 (4.35) |
| XY + IN | 0.80 ± 0.01 (0.81) | 9.28 ± 0.15 (9.52) | 0.51 ± 0.01 (0.52) | 3.74 ± 0.18 (4.01) |
| XY + TN | 0.81 ± 0.01 (0.81) | 10.71 ± 0.08 (10.80) | 0.58 ± 0.01 (0.59) | 5.04 ± 0.10 (5.17) |
| XY + DPE | 0.797 ± 0.001 (0.80) | 10.48 ± 0.13 (10.56) | 0.597 ± 0.003 (0.60) | 4.84 ± 0.11 (5.01) |
| XY + DBE | 0.80 ± 0.01 (0.79) | 9.78 ± 0.15 (9.93) | 0.57 ± 0.01 (0.57) | 4.40 ± 0.07 (4.49) |
| CF + DIO | 0.77 ± 0.01 (0.77) | 9.03 ± 0.24 (9.27) | 0.45 ± 0.01 (0.47) | 3.17 ± 0.18 (3.37) |
| Light Intensity (mW/cm2) | ||||||
|---|---|---|---|---|---|---|
| 100 | 82 | 43 | 26 | 10 | ||
| XY | Charge density (×1017 cm−3) | 6.77 | 6.70 | 6.60 | 6.56 | 6.44 |
| Lifetime (μs) | 4.57 | 4.78 | 7.06 | 8.95 | 18.68 | |
| XY + TMB | Charge density (×1017 cm−3) | 5.27 | 5.24 | 5.15 | 5.10 | 5.05 |
| Lifetime (μs) | 5.33 | 5.40 | 6.14 | 7.62 | 11.29 | |
| XY + IN | Charge density (×1017 cm−3) | 4.45 | 4.40 | 4.33 | 4.32 | 4.31 |
| Lifetime (μs) | 4.25 | 5.15 | 9.27 | 11.60 | 13.71 | |
| XY + TN | Charge density (×1017 cm−3) | 8.63 | 8.58 | 8.47 | 8.43 | 8.42 |
| Lifetime (μs) | 6.69 | 7.36 | 10.98 | 14.63 | 23.76 | |
| XY + DPE | Charge density (×1017 cm−3) | 5.37 | 5.35 | 5.27 | 5.21 | 5.18 |
| Lifetime (μs) | 6.69 | 7.09 | 8.89 | 11.23 | 16.75 | |
| XY + DBE | Charge density (×1017 cm−3) | 5.65 | 5.62 | 5.51 | 5.49 | 5.43 |
| Lifetime (μs) | 3.48 | 4.18 | 6.16 | 8.45 | 13.84 | |
| Solvent | μh (cm2·V−1·s−1) | μe (cm2·V−1·s−1) | μh/μe |
|---|---|---|---|
| XY | 1.08 (±0.11) × 10−4 | 1.87 (±1.1) × 10−6 | 57.7 |
| XY + TMB | 2.68 (±0.98) × 10−5 | 1.29 (±0.88) × 10−5 | 2.08 |
| XY + IN | 1.67 (±0.73) × 10−5 | 9.40 (±2.5) × 10−6 | 1.77 |
| XY + TN | 3.86 (±0.53) × 10−5 | 5.95 (±1.1) × 10−6 | 6.49 |
| XY + DPE | 1.51 (±0.16) × 10−4 | 4.35 (±0.88) × 10−6 | 34.7 |
| XY + DBE | 1.04 (±0.13) × 10−4 | 7.18 (±3.1) × 10−6 | 14.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Choi, H.; Lee, M.; Jung, H.; Shin, Y.; Lee, S.; Kim, K.; Kim, M.H.; Kwak, K.; Kim, B. Critical Role of Non-Halogenated Solvent Additives in Eco-Friendly and Efficient All-Polymer Solar Cells. Polymers 2023, 15, 1354. https://doi.org/10.3390/polym15061354
Kim S, Choi H, Lee M, Jung H, Shin Y, Lee S, Kim K, Kim MH, Kwak K, Kim B. Critical Role of Non-Halogenated Solvent Additives in Eco-Friendly and Efficient All-Polymer Solar Cells. Polymers. 2023; 15(6):1354. https://doi.org/10.3390/polym15061354
Chicago/Turabian StyleKim, Saeah, Huijeong Choi, Myeongjae Lee, Hyeseung Jung, Yukyung Shin, Seul Lee, Kyungkon Kim, Myung Hwa Kim, Kyungwon Kwak, and BongSoo Kim. 2023. "Critical Role of Non-Halogenated Solvent Additives in Eco-Friendly and Efficient All-Polymer Solar Cells" Polymers 15, no. 6: 1354. https://doi.org/10.3390/polym15061354