Click Synthesis of Triazole Polymers Based on Lignin-Derived Metabolic Intermediate and Their Strong Adhesive Properties to Cu Plate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
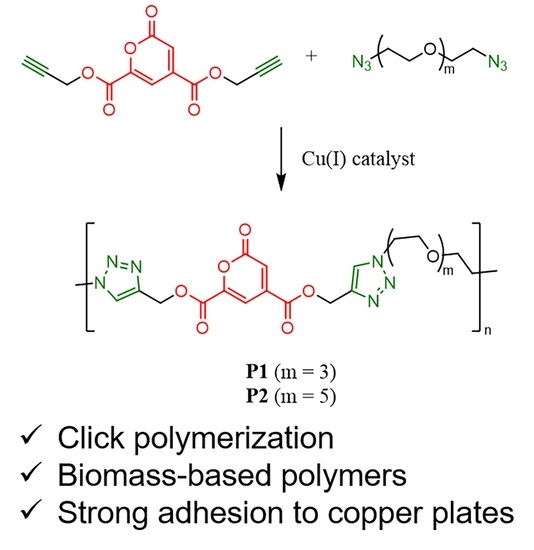
2.3. Synthesis
3. Results
3.1. Polymer Synthesis and Characterization
3.2. Thermal Properties
3.3. Adhesive Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sust. Energ. Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Vink, E.T.; Rábago, K.R.; Glassner, D.A.; Gruber, P.R. Applications of life cycle assessment to NatureWorks™ polylactide (PLA) production. Polym. Degrad. Stab. 2003, 80, 403–419. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A. Polymers from Renewable Resources: A Challenge for the Future of Macromolecular Materials. Macromolecules 2008, 41, 9491–9504. [Google Scholar] [CrossRef]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef]
- Changzhi, L.; Xiaochen, Z.; Aiqin, W.; George, W.H.; Tao, Z. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Azadi, P.; Inderwildi, O.R.; Farnood, R.; King, D.A. Liquid fuels, hydrogen and chemicals from lignin: A critical review. Renew. Sust. Energ. Rev. 2013, 21, 506–523. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuomela, M.; Vikman, M.; Hatakka, A.; Itävaara, M. Biodegradation of lignin in a compost environment: A review. Bioresour. Technol. 2000, 72, 169–183. [Google Scholar] [CrossRef]
- Bugg, T.D.; Ahmad, M.; Hardiman, E.M.; Rahmanpour, R. Pathways for degradation of lignin in bacteria and fungi. Nat. Prod. Rep. 2011, 28, 1883–1896. [Google Scholar] [CrossRef]
- Grzegorz, J.; Anna, P.; Justyna, S.; Urszula, Ś.; Anna, J.; Andrzej, P. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Dueñas, F.J.; Martínez, Á.T. Microbial degradation of lignin: How a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this. Microb. Biotechnol. 2009, 2, 164–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.E.; Chang, M.C. Exploring bacterial lignin degradation. Curr. Opin. Chem. Biol. 2014, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Leonowicz, A.; Matuszewska, A.; Luterek, J.; Ziegenhagen, D.; Wojtaś-Wasilewska, M.; Cho, N.; Hofrichter, M.; Rogalski, J. Biodegradation of Lignin by White Rot Fungi. Fungal Genet. Biol. 1999, 27, 175–185. [Google Scholar] [CrossRef]
- Otsuka, Y.; Araki, T.; Suzuki, Y.; Nakamura, M.; Kamimura, N.; Masai, E. High-Level Production of 2-Pyrone-4,6-dicarboxylic Acid from a Lignin-Derived Aromatic Compound by Metabolically Engineered Fermentation to Realize Industrial Valorization Processes of Lignin. Available online: https://ssrn.com/abstract=4352741 (accessed on 1 February 2023).
- Michinobu, T.; Bito, M.; Yamada, Y.; Katayama, Y.; Noguchi, K.; Masai, E.; Nakamura, M.; Ohara, S.; Shigehara, K. Molecular properties of 2-pyrone-4,6-dicarboxylic acid (PDC) as a stable metabolic intermediate of lignin isolated by fractional precipitation with Na+ ion. Bull. Chem. Soc. Jpn. 2007, 80, 2436–2442. [Google Scholar] [CrossRef]
- Michinobu, T.; Hishida, M.; Sato, M.; Katayama, Y.; Masai, E.; Nakamura, M.; Otsuka, Y.; Ohara, S.; Shigehara, K. Polyesters of 2-pyrone-4,6-dicarboxylic acid (PDC) obtained from a metabolic intermediate of lignin. Polym. J. 2008, 40, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Hishida, M.; Shikinaka, K.; Katayama, Y.; Kajita, S.; Masai, E.; Nakamura, M.; Otsuka, Y.; Ohara, S.; Shigehara, K. Polyesters of 2-pyrone-4,6-dicarboxylic acid (PDC) as bio-based plastics exhibiting strong adhering properties. Polym. J. 2009, 41, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Michinobu, T.; Bito, M.; Yamada, Y.; Tanimura, M.; Katayama, Y.; Masai, E.; Nakamura, M.; Otsuka, Y.; Ohara, S.; Shigehara, K. Fusible, elastic, and biodegradable polyesters of 2-pyrone-4,6-dicarboxylic acid (PDC). Polym. J. 2009, 41, 1111–1116. [Google Scholar] [CrossRef]
- Michinobu, T.; Bito, M.; Tanimura, M.; Katayama, Y.; Masai, E.; Nakamura, M.; Otsuka, Y.; Ohara, S.; Shigehara, K. Synthesis and characterization of hybrid biopolymers of L-lactic acid and 2-pyrone-4,6-dicarboxylic acid. J. Macromol. Sci. A 2010, 47, 564–570. [Google Scholar] [CrossRef]
- Michinobu, T.; Hiraki, K.; Fujii, N.; Shikinaka, K.; Katayama, Y.; Masai, E.; Nakamura, M.; Otsuka, Y.; Ohara, S.; Shigehara, K. Organogels of lignin-derived stable metabolic intermediate, 2-pyrone-4,6-dicarboxylic acid (PDC), bearing cholesteryl groups. Chem. Lett. 2010, 39, 400–401. [Google Scholar] [CrossRef]
- Michinobu, T.; Hiraki, K.; Fujii, N.; Shikinaka, K.; Katayama, Y.; Masai, E.; Nakamura, M.; Otsuka, Y.; Ohara, S.; Shigehara, K. Liquid crystallinity and organogelation behavior of lignin-derived metabolic intermediate bearing cholesterol groups. Bull. Chem. Soc. Jpn. 2011, 84, 667–674. [Google Scholar] [CrossRef]
- Shikinaka, K.; Hashimoto, Y.; Kajita, S.; Masai, E.; Katayama, Y.; Nakamura, M.; Otsuka, Y.; Ohara, S.; Shigehara, K. Thermoplastic polyesters of 2-pyrone-4,6-dicarboxylic acid (PDC) obtained from a metabolic intermediate of lignin. Sen’i Gakkaishi 2013, 69, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Michinobu, T.; Hiraki, K.; Inazawa, Y.; Katayama, Y.; Masai, E.; Nakamura, M.; Ohara, S.; Shigehara, K. Click synthesis and adhesive properties of novel biomass-based polymers from lignin-derived stable metabolic intermediate. Polym. J. 2011, 43, 648–653. [Google Scholar] [CrossRef]
- Cheng, Y.; Kuboyama, K.; Akasaka, S.; Araki, T.; Masai, E.; Nakamura, M.; Michinobu, T. Polyurethanes based on lignin-derived metabolic intermediate with strong adhesion to metals. Polym. Chem. 2022, 13, 6589–6598. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Meldal, M. Polymer “Clicking” by CuAAC Reactions. Macromol. Rapid Commun. 2008, 29, 1016–1051. [Google Scholar] [CrossRef]
- Qin, A.; Lam, J.W.Y.; Tang, B.Z. Click polymerization. Chem. Soc. Rev. 2010, 39, 2522–2544. [Google Scholar] [CrossRef] [Green Version]
- Nagao, Y.; Takasu, A. “Click polyester”: Synthesis of polyesters containing triazole units in the main chain via safe and rapid “click” chemistry and their properties. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4207–4218. [Google Scholar] [CrossRef]
- Wang, Y.; Michinobu, T. Polymeric chemosensors: A conventional platform with new click chemistry. Bull. Chem. Soc. Jpn. 2017, 90, 1388–1400. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Michinobu, T. Postpolymerization modification: A powerful tool for the synthesis and function tuning of stimuli-responsive polymers. Macromol. Chem. Phys. 2022, 223, 2100370. [Google Scholar] [CrossRef]
- Ross, D.A.W.; Findlay, J.A.; Vasdev, R.A.S.; Crowley, J.D. Can 2-pyridyl-1,2,3-triazole “click” ligands be used to develop Cu(I)/Cu(II) molecular switches? ACS Omega 2021, 6, 30115–30129. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Shikinaka, K.; Katayama, Y.; Kajita, S.; Masai, E.; Nakamura, M.; Otsuka, Y.; Ohara, S.; Shigehara, K. Tenacious epoxy adhesives prepared from lignin-derived stable metabolic intermediate. Sen’i Gakkaishi 2009, 65, 359–362. [Google Scholar] [CrossRef] [Green Version]
- Ishida, H.; Johnson, R. The inhibition of copper corrosion by azole compounds in humid environments. Corros. Sci. 1986, 26, 657–667. [Google Scholar] [CrossRef]
- Breugst, M.; Reissig, H.-U. The Huisgen reaction: Milestones of the 1,3-dipolar cycloaddition. Angew. Chem. Int. Ed. 2020, 59, 12293–12307. [Google Scholar] [CrossRef] [Green Version]
- Bito, M.; Michinobu, T.; Katayama, Y.; Otsuka, Y.; Nakamura, M.; Ohara, S.; Masai, E.; Shigehara, K. 2-Pyranone-4, 6-dicarboxylic acid as a source of green-plastics and anti-bacterial chemicals. Trans. Mater. Res. Soc. Jpn. 2008, 33, 1165–1168. [Google Scholar] [CrossRef] [Green Version]
- Díaz, D.D.; Punna, S.; Holzer, P.; McPherson, A.K.; Sharpless, K.B.; Fokin, V.V.; Finn, M.G. Click chemistry in materials synthesis. 1. Adhesive polymers from copper-catalyzed azide-alkyne cycloaddition. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 4392–4403. [Google Scholar] [CrossRef]





| Tensile Lap Shear Strength (MPa) | ||||
|---|---|---|---|---|
| 100 °C | 150 °C | 200 °C | 250 °C | |
| P1 (1 + 2)-Cu | - | 1.06 | 3.05 | - |
| P1 (1 + 2)-Fe | - | - | - | - |
| P2 (1 + 3)-Cu | - | 1.90 | 4.18 | - |
| P2 (1 + 3)-Fe | - | - | 0.48 | - |
| Tensile Lap Shear Strength (MPa) | |
|---|---|
| P1-Cu | 5.56 |
| P2-Cu | 5.73 |
| P1-Fe | 4.17 |
| P2-Fe | 2.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Joshi, M.; Araki, T.; Kamimura, N.; Masai, E.; Nakamura, M.; Michinobu, T. Click Synthesis of Triazole Polymers Based on Lignin-Derived Metabolic Intermediate and Their Strong Adhesive Properties to Cu Plate. Polymers 2023, 15, 1349. https://doi.org/10.3390/polym15061349
Jin Y, Joshi M, Araki T, Kamimura N, Masai E, Nakamura M, Michinobu T. Click Synthesis of Triazole Polymers Based on Lignin-Derived Metabolic Intermediate and Their Strong Adhesive Properties to Cu Plate. Polymers. 2023; 15(6):1349. https://doi.org/10.3390/polym15061349
Chicago/Turabian StyleJin, Yijie, Manjusha Joshi, Takuma Araki, Naofumi Kamimura, Eiji Masai, Masaya Nakamura, and Tsuyoshi Michinobu. 2023. "Click Synthesis of Triazole Polymers Based on Lignin-Derived Metabolic Intermediate and Their Strong Adhesive Properties to Cu Plate" Polymers 15, no. 6: 1349. https://doi.org/10.3390/polym15061349