Superparamagnetic Multifunctionalized Chitosan Nanohybrids for Efficient Copper Adsorption: Comparative Performance, Stability, and Mechanism Insights
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Adsorbents
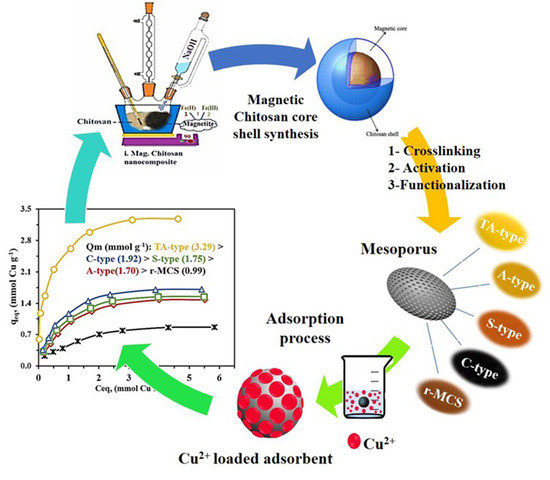
2.2.1. Preparation of Activated Crosslinked Chitosan−Magnetite Nanohybrid
2.2.2. Nanohybrid Functionalization
2.3. Characterization Techniques
2.4. Adsorption Experiments
3. Results and Discussion
3.1. Fabrication Mechanism
3.2. Materials Characterization
3.2.1. Physical Characterization
Nano-Structure Characterization—HR-TEM Analysis
Crystalline Structure—XRD Analysis
Textural Properties—BET Surface Analysis
Thermogravimetric Analysis (TGA)
Magnetic Properties—VSM Analysis
3.2.2. Chemical Characterization
XPS Spectroscopy
Element Analysis-CHNS/O
FTIR Spectroscopy
pHZPC—Drift Titration
Chemical Analysis—Semi-Quantitative EDX
3.3. Adsorption Investigations
3.3.1. Effect of Initial pH
3.3.2. Adsorption Time Effect and Kinetic Studies
3.3.3. Initial Cu(II) Concentration Effect
3.3.4. Temperature Effect and Thermodynamic Parameters
3.3.5. Adsorbent Reusability
3.3.6. Chemical Stability Examination
3.3.7. Metal Adsorption Interaction and Complexation
3.3.8. Adsorption Selectivity
3.3.9. Direction of a Relationship between Variables
3.4. Simulation and Graphical Mathematical Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Saydeh, S.; El-Naas, M.; Zaidi, S. Copper removal from industrial wastewater: A comprehensive review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Donia, A.; Atia, A.; Abouzayed, F. Preparation and characterization of nano-magnetic cellulose with fast kinetic properties towards the adsorption of some metal ions. Chem. Eng. J. 2012, 191, 22–30. [Google Scholar] [CrossRef]
- Bian, P.; Liu, Y.; Zheng, X.; Shen, W. Removal and mechanism of cadmium, lead and copper in water by functional modification of Silkworm excrement biochar. Polymers 2022, 14, 2889. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.D.R. Removal of copper(II) from aqueous solution using zinc oxide nanoparticle impregnated mixed matrix hollow fiber membrane. Environ. Technol. Innov. 2022, 26, 102300. [Google Scholar] [CrossRef]
- Elgarahy, A.; Elwakeel, K.; Mohammad, S.; Elshoubaky, G. Multifunctional eco-friendly sorbent based on marine brown algae and bivalve shells for subsequent uptake of Congo red dye and copper(II) ions. J. Environ. Chem. Eng. 2020, 8, 103915. [Google Scholar] [CrossRef]
- Baraka, A.; Hall, P.; Heslop, M. Preparation and characterization of melamine–formaldehyde–DTPA chelating resin and its use as an adsorbent for heavy metals removal from wastewater. React. Funct. Polym. 2007, 67, 585–600. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, B.; Jia, L.; Qiao, X.; Li, Z. Study on adsorption mechanism of mercury on Ce-Cu modified iron-based biochar. Chem. Eng. J. Adv. 2022, 10, 100259. [Google Scholar] [CrossRef]
- Taylor, A.; Tsuji, J.; Garry, M.; McArdle, M.; Goodfellow, W.; Adams, W.; Menzie, C. Critical review of exposure and effects: Implications for setting regulatory health criteria for ingested copper. Environ. Manag. 2020, 65, 131–159. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Musharraf, S.; Bhanger, M.; Malik, M. Salicylaldehyde derivative of nano-chitosan as an efficient adsorbent for lead(II), copper(II), and cadmium(II) ions. Int. J. Biol. Macromol. 2020, 147, 643–652. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Vieira, R.; Oliveira, M.; Guibal, E.; Rodríguez-Castellón, E.; Beppu, M. Copper, mercury and chromium adsorption on natural and crosslinked chitosan films: An XPS investigation of mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2011, 374, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Neiber, R.; Galhoum, A.; El Sayed, I.E.-T.; Guibal, E.; Xin, J.; Lu, X. Selective lead (II) sorption using aminophosphonate-based sorbents: Effect of amine linker, characterization and sorption performance. Chem. Eng. J. 2022, 442, 136300. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.; Menya, E.; Kalibbala, H. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Kayalvizhi, K.; Alhaji, N.; Saravanakkumar, D.; Mohamed, S.; Kaviyarasud, K.; Ayeshamariam, A.; Al-Mohaimeed, A.; AbdelGawwad, M.; Elshikh, M. Adsorption of copper and nickel by using sawdust chitosan nanocomposite beads—A kinetic and thermodynamic study. Enviro. Res. 2022, 203, 111814. [Google Scholar] [CrossRef]
- Tan, T.; Somat, H.; Latif, M.; Rashid, S. One-pot solvothermal synthesis of Zr-based MOFs with enhanced adsorption capacity for Cu2+ ions removal. J. Solid State Chem. 2022, 15, 123429. [Google Scholar] [CrossRef]
- Zidan, T.; Abdelhamid, A.; Zaki, E. N-Aminorhodanine modified chitosan hydrogel for antibacterial and copper ions removal from aqueous solutions. Int. J. Biol. Macromol. 2020, 158, 32–42. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Ghaedi, A.; Panahimehr, M.; Nejad, A.; Hosseini, S.; Vafaei, A.; Baneshi, M. Factorial experimental design for the optimization of highly selective adsorption removal of lead and copper ions using metal organic framework MOF-2 (Cd). J. Mol. Liq. 2018, 272, 15–26. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, S.; Tian, Z.; Duan, G.; Pan, H.; Yue, Y.; Li, S.; Jian, S.; Yang, W.; Liu, K.; et al. MOFs meet wood: Reusable magnetic hydrophilic composites toward efficient water treatment with super-high dye adsorption capacity at high dye concentration. Chem. Eng. J. 2022, 446, 136851. [Google Scholar] [CrossRef]
- Cuong, D.; Liu, N.-L.; Nguyen, V.; Hou, C.-H. Meso/micropore-controlled hierarchical porous carbon derived from activated biochar as a high-performance adsorbent for copper removal. Sci. Total Environ. 2019, 692, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Galhoum, A.; Akashi, T.; Linnolahti, M.; Hirvi, J.; Al-Sehemid, A.; Kalam, A.; Guibal, E. Functionalization of poly(glycidylmethacrylate) with iminodiacetate and imino phosphonate groups for enhanced sorption of neodymium—Sorption performance and molecular modeling. React. Funct. Polym. 2022, 180, 105389. [Google Scholar] [CrossRef]
- Galhoum, A.; Eisa, W.; El-Sayed, I.E.-T.; Tolba, A.; Shalaby, Z.; Mohamady, S.; Muhammad, S.; Hussien, S.; Akashi, T.; Guibal, E. A new route for manufacturing poly(aminophosphonic)-functionalized poly(glycidyl methacrylate)-magnetic nanocomposite-Application to uranium sorption from ore leachate. Environ. Pollut. 2020, 264, 114797. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, A.; Galhoum, A.; Alshahrie, A.; Al-Turki, Y.; Al-Amri, A.; Wageh, S. Mechanistic studies of uranyl interaction with functionalized mesoporous chitosan-superparamagnetic nanocomposites for selective sorption: Characterization and sorption performance. Mater. Today Commun. 2022, 33, 104536. [Google Scholar] [CrossRef]
- Imam, E.; El-Sayed, I.E.-T.; Mahfouz, M.; Tolba, A.; Akashi, T.; Galhoum, A.; Guibal, E. Synthesis of α-aminophosphonate functionalized chitosan sorbents: Effect of methyl vs phenyl group on uranium sorption. Chem. Eng. J. 2018, 352, 1022–1034. [Google Scholar] [CrossRef]
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-based (nano)materials for novel biomedical applications. Molecules 2019, 24, 1960. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Shapter, J.; Popelka-Filcoff, R.; Bennett, J.; Ellis, A. Copper removal using bio-inspired polydopamine coated natural zeolites. J. Hazard. Mat. 2014, 273, 174–182. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Benettayeb, A.; Morsli, A.; Elwakeel, K.; Hamza, M.; Guibal, E. Recovery of heavy metal ions using magnetic glycine-modified chitosan—Application to aqueous solutions and tailing leachate. Appl. Sci. 2021, 11, 8377. [Google Scholar] [CrossRef]
- Yamaura, M.; Camilo, R.; Sampaio, L.; Macêdo, M.; Nakamura, M.; Toma, H. Preparation and characterization of (3-aminopropyl)triethoxysilane-coated magnetite nanoparticles. J. Magn. Magn. Mater. 2004, 279, 210–217. [Google Scholar] [CrossRef]
- Galhoum, A.; Mahfouz, M.; Gomaa, N.; Vincent, T.; Guibal, E. Chemical modifications of chitosan nano-based magnetic particles for enhanced uranyl sorption. Hydrometallurgy 2017, 168, 127–134. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, W.; Pan, B.; Zhang, Q.; Zhang, W.; Lv, L. Facile Fabrication of Magnetic Chitosan Beads of Fast Kinetics and High Capacity for Copper Removal. ACS Appl. Mater. Interfaces 2014, 6, 3421–3426. [Google Scholar] [CrossRef]
- Pourmortazavi, S.; Sahebi, H.; Zandavar, H.; Mirsadeghi, S. Fabrication of Fe3O4 nanoparticles coated by extracted shrimp peels chitosan as sustainable adsorbents for removal of chromium contaminates from wastewater: The design of experiment. Compos. B Eng. 2019, 175, 107130. [Google Scholar] [CrossRef]
- Morshedy, A.; Galhoum, A.; Aleem, A.A.H.A.; El-din, M.S.; Okaba, D.; Mostafa, M.; Mira, H.; Yang, Z.; El-Sayed, I.E. Functionalized aminophosphonate chitosan-magnetic nanocomposites for Cd(II) removal from aqueous solutions: Performance and mechanisms of sorption. Appl. Surf. Sci. 2021, 561, 150069. [Google Scholar] [CrossRef]
- Galhoum, A.; Atia, A.; Mahfouz, M.; Abdel-Rehem, S.; Gomaa, N.; Vincent, T.; Guibal, E. Dy(III) recovery from dilute solutions using magnetic-chitosan nano-based particles grafted with amino acids. J. Mater. Sci. 2015, 50, 2832–2848. [Google Scholar] [CrossRef]
- Esfandiari, N.; Kashefi, M.; Mirjalili, M.; Afsharnezhad, S. Role of silica mid-layer in thermal and chemical stability of hierarchical Fe3O4-SiO2-TiO2 nanoparticles for improvement of lead adsorption: Kinetics, thermodynamic and deep XPS investigation. Mater. Sci. Eng. B. 2020, 262, 114690. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.; Galhoum, A.; Alshahrie, A.; Al-Turki, Y.; Al-Amri, A.; Wageh, S. Mesoporous magnetic cysteine functionalized chitosan nanocomposite for selective uranyl ions sorption: Experimental, structural characterization, and mechanistic studies. Polymers 2022, 14, 2568. [Google Scholar] [CrossRef]
- Alghamdi, N.A. Mesoporous magnetic-polyaminated-chitosan nanocomposite for selective uranium removal: Performance and mechanistic studies. Int. J. Environ. Sci. Technol. 2022, 2022, 1–22. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.; Haron, M.; Namvar, F.; Nadi, B.; Rahman, M.; Amin, J. Synthesis, Surface Modification and Characterisation of Biocompatible Magnetic Iron Oxide Nanoparticles for Biomedical Applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef] [Green Version]
- Galhoum, A.; Mafhouz, M.; Abdel-Rehem, S.; Gomaa, N.; Atia, A.; Vincent, T.; Guibal, E. Cysteine-Functionalized Chitosan Magnetic Nano-Based Particles for the Recovery of Light and Heavy Rare Earth Metals: Uptake Kinetics and Sorption Isotherms. Nanomaterials 2015, 5, 154–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Wang, Y.; Wang, Q.; Wu, J.; Duan, G.; Xu, W.; Jian, S. Magnetically separable and recyclable Fe3O4@PDA covalent grafted by l-cysteine core-shell nanoparticles toward efficient removal of Pb2+. Vacuum 2021, 189, 110229. [Google Scholar] [CrossRef]
- Stoia, M.; Istratie, R.; Păcurariu, C. Investigation of magnetite nanoparticles stability in air by thermal analysis and FTIR spectroscopy. J. Therm. Anal. Calorim. 2016, 125, 1185–1198. [Google Scholar] [CrossRef]
- Shahrashoub, M.; Bakhtiari, S.; Afroosheh, F.; Googheri, M. Recovery of iron from direct reduction iron sludge and biosynthesis of magnetite nanoparticles using green tea extract. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126675. [Google Scholar] [CrossRef]
- Zhang, X.; Jiao, C.; Wang, J.; Liu, Q.; Li, R.; Yang, P.; Zhang, M. Removal of uranium(VI) from aqueous solutions by magnetic Schiff base: Kinetic and thermodynamic investigation. Chem. Eng. J. 2012, 198–199, 412–419. [Google Scholar] [CrossRef]
- Kong, H.; Song, J.; Jang, J. One-step fabrication of magnetic γ-Fe2O3/polyrhodanine nanoparticles using in situ chemical oxidation polymerization and their antibacterial properties. Chem. Comm. 2010, 46, 6735–6737. [Google Scholar] [CrossRef]
- El-Magied, M.A.; Galhoum, A.; Atia, A.; Tolba, A.; Maize, M.; Vincent, T.; Guibal, E. Cellulose and chitosan derivatives for enhanced sorption of erbium(III). Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 580–593. [Google Scholar] [CrossRef]
- Sharma, J.; Srivastava, P.; Singh, G.; Virk, H. Nanoferrites of Transition Metals and Their Catalytic Activity. Diffus. Defect Data Pt. B Solid State Phenom. 2016, 241, 126–138. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- The International XPS Database of XPS Reference Spectra, Peak-Fits & Six (6) BE Tables. Available online: https://xpsdatabase.com/ (accessed on 16 October 2020).
- Grosvenor, A.; Kobe, B.; Biesinger, M.; McIntyre, N. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Wang, J.; Ma, X.; Qu, F.; Asiri, A.; Sun, X. Fe-Doped Ni2P Nanosheet Array for High-Efficiency Electrochemical Water Oxidation. Inorg. Chem. 2017, 56, 1041–1044. [Google Scholar] [CrossRef]
- Oh, S.; Yoo, D.; Shin, Y.; Kim, H.; Kim, H.; Chung, Y.; Park, W.; Youk, J. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra: Apractical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd: Chochester, UK, 2000. [Google Scholar]
- Muthuselvi, C.; Pandiarajan, S.; Ravikumar, B.; Athimoolam, S.; Srinivasan, N.; Krishnakumar, R. FT-IR and FT-Raman spectroscopic analyzes of indeno quinoxaline derivative crystal. Asian J. Appl. Sci. 2018, 11, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Willianms, R. Pka Data Compiled by R. Willianms. 2017, pp. 1–33. Available online: https://organicchemistrydata.org/hansreich/resources/pka/pka_data/pka-compilation-williams.pdf{pKa_compilation-1-Williams.pdf} (accessed on 25 August 2020).
- Fraga, T.; de Lima, L.; de Souza, Z.; Carvalho, M.; Freire, E.; Ghislandi, M.; da Motta, M. Amino-Fe3O4-functionalized graphene oxide as a novel adsorbent of Methylene Blue: Kinetics, equilibrium, and recyclability aspects. Environ. Sci. Pollut. Res. 2019, 26, 28593–28602. [Google Scholar] [CrossRef]
- Thompson, A.; Attwood, D.; Gullikson, E.; Howells, M.; Kim, K.-J.; Kirz, J.; Kortright, J.; Lindau, I.; Liu, Y.; Pianetta, P.; et al. X-Ray Data Booklet; Lawrence Berkeley National Laboratory—University of California: Berkeley, CA, USA, 2009. [Google Scholar]
- Fouda, S.; El-Sayed, I.; Attia, N.; Abdeen, M.; Aleem, A.A.; Nassar, I.; Mira, H.; Gawad, E.; Kalam, A.; Al-Ghamdi, A.; et al. Mechanistic study of Hg(II) interaction with three different α-aminophosphonate adsorbents: Insights from batch experiments and theoretical calculations. Chemosphere 2022, 304, 135253. [Google Scholar] [CrossRef]
- Persson, I. Hydrated metal ions in aqueous solution: How regular are their structures? Pure Appl. Chem. 2010, 82, 1901–1917. [Google Scholar] [CrossRef]
- Tien, C. Adsorption Calculations and Modeling; Butterworth-Heinemann: Boston, MA, USA, 1994. [Google Scholar]
- Hu, X.; Chen, C.; Zhang, D.; Xue, Y. Kinetics, isotherm and chemical speciation analysis of Hg(Ⅱ) adsorption over oxygen-containing MXene adsorbent. Chemosphere 2021, 278, 130206. [Google Scholar] [CrossRef]
- Hubbe, M.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. BioResources 2019, 14, 7582–7626. [Google Scholar] [CrossRef]
- Doğan, M.; Özdemir, Y.; Alkan, M. Adsorption kinetics and mechanism of cationic methyl violet and methylene blue dyes onto sepiolite. Dyes Pigm 2007, 75, 701–713. [Google Scholar] [CrossRef]
- Zhang, G.; Fang, Y.; Wang, Y.; Liu, L.; Mei, D.; Ma, F.; Meng, Y.; Dong, H.; Zhang, C. Synthesis of amino acid modified MIL-101 and efficient uranium adsorption from water. J. Mol. Liq. 2022, 349, 118095. [Google Scholar] [CrossRef]
- Chen, H.; Lin, J.; Zhang, N.; Chen, L.; Zhong, S.; Wang, Y.; Zhang, W.; Ling, Q. Preparation of MgAl-EDTA-LDH based electrospun nanofiber membrane and its adsorption properties of copper(II) from wastewater. J. Hazard. Mat. 2018, 345, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Wang, J.; Sun, T.; Sharaf, F.; Haris, M.; Lei, S. Enhanced and selective adsorption of Copper ions from acidic conditions by diethylenetriaminepentaacetic acid-chitosan sewage sludge composite. J. Environ. Chem. Eng. 2020, 8, 104430. [Google Scholar] [CrossRef]
- Tan, T.; Mo, K.; Lai, S.; Ling, T.-C. Investigation on the copper ion removal potential of a facile-fabricated foamed geopolymer sphere for wastewater remediation. Clean. Mater. 2022, 4, 100088. [Google Scholar] [CrossRef]
- Al-Ghouti, M.; Da, D. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Rangabhashiyam, S.; Anu, N.; Nandagopal, M.G.; Selvaraju, N. Relevance of isotherm models in biosorption of pollutants by agricultural byproducts. J. Environ. Chem. Eng. 2014, 2, 398–414. [Google Scholar] [CrossRef]
- Lima, E.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Xia, D.; Liu, Y.; Cheng, X.; Gu, P.; Chen, Q.; Zhang, Z. Temperature-tuned fish-scale biochar with two-dimensional homogeneous porous structure: A promising uranium extractant. Appl. Surf. Sci. 2022, 591, 153136. [Google Scholar] [CrossRef]
- Aslani, C.; Amik, O. Active Carbon/PAN composite adsorbent for uranium removal: Modeling adsorption isotherm data, thermodynamic and kinetic studies. Appl. Radiat. Isot. 2021, 168, 109474. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Z.; Yuan, D.; Wang, Y.; Dai, Y.; Zhu, Y.; Chew, J. Introduction of amino groups into polyphosphazene framework supported on CNT and coated Fe3O4 nanoparticles for enhanced selective U(VI) adsorption. Appl. Surf. Sci. 2019, 466, 893–902. [Google Scholar] [CrossRef]
- Zhai, Q.-Z. Use of SBA-15 ordered nano mesoporous silica for removal of copper(II) from aqueous media: Studies on equilibrium, isotherm, kinetics and thermodynamics. J. Environ. Chem. Eng. 2019, 7, 103069. [Google Scholar] [CrossRef]
- Ertl, G.; Hierl, R.; Knözinger, H.; Thiele, N.; Urbach, H. XPS study of copper aluminate catalysts. Appl. Surf. Sci. 1980, 5, 49–64. [Google Scholar] [CrossRef]
- Borsari, M. Cadmium: Coordination Chemistry. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Wiley Online Library: Hoboken, NJ, USA, 2011; pp. 1–16. [Google Scholar]
- Pearson, R.G. Acids and bases. Science 1966, 151, 172–177. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J. Correlating metal ionic characteristics with biosorption capacity using QSAR model. Chemosphere 2007, 69, 1610–1616. [Google Scholar]
- Boyadjiev, C. Theoretical Chemical Engineering: Modeling and Simulation; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
















| Adsorbent | Operating Condition (pH0, Time, Temp. (in °C), SD (g.L−1)) | qm, mmol Cu.g−1 | Ref. |
|---|---|---|---|
| Pristine natural zeolite | 5.5, 24 h, 26 °C, 1.0 | 0.235 | [28] |
| PDA treated zeolite powders | 0.45 | ||
| Zr-based MOFs (MOF-801) | 5.5, 180 min, 27 °C, 0.4 | 0.278 | [16] |
| DTPA modified sludge | 3.0, 60 min, 25 °C, 0.4 | 0.557 | [67] |
| Foamed geopolymer sphere | 5.0, 48 h, 27 °C, 1.0 | 0.596 | [68] |
| CS-GLA | 5.0, 360 min, 26 °C, 5.0 | 0.601 | [17] |
| Aminorhodanin@CS | 5.0, 20 min, 26 °C, 5.0 | 0.984 | |
| Melamine–HCHO–DTPA | 4.5, 60 min, 20 °C, 10.0 | 0.729 | [7] |
| N-Ch-Sal | 5.0, 120 min, 26 °C, 1.0 | 1.331 | [10] |
| Mag. Cel-DETA (MCGT) | 5.4, 5 min, 25 °C, 1.0 | 1.449 | [18] |
| Silkworm excrement biochar | 5.0, 24 h, 25 °C, 1.0 | 1.471 | [4] |
| MgAl-EDTA-LDH | 5.0, 60 min, 25 °C, 1.25 | 1.901 | [66] |
| Meso/microporous Carbon | 5.0, 120 min, 25 °C, 0.35 | 4.134 | [21] |
| Cd-terephthalate-MOF-2 | 6.0, 60 min, 27 °C, 0.5 | 6.842 | [19] |
| CR dye@Urea Ca-alginate | 6.5, 90 min, 55 °C, 1.5 | 6.94 | [6] |
| r-MCS | 5.0, 240 min, 55 °C, 0.5 | 0.987 | Here |
| A-type | 5.0, 90 min, 55 °C, 0.5 | 1.696 | |
| S-typr | 5.0, 60 min, 55 °C, 0.5 | 1.745 | |
| C-type | 5.0, 50 min, 55 °C, 0.5 | 1.921 | |
| TA-type | 5.0, 30 min, 25 °C, 0.5 | 3.287 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ghamdi, A.A.; Galhoum, A.A.; Alshahrie, A.; Al-Turki, Y.A.; Al-Amri, A.M.; Wageh, S. Superparamagnetic Multifunctionalized Chitosan Nanohybrids for Efficient Copper Adsorption: Comparative Performance, Stability, and Mechanism Insights. Polymers 2023, 15, 1157. https://doi.org/10.3390/polym15051157
Al-Ghamdi AA, Galhoum AA, Alshahrie A, Al-Turki YA, Al-Amri AM, Wageh S. Superparamagnetic Multifunctionalized Chitosan Nanohybrids for Efficient Copper Adsorption: Comparative Performance, Stability, and Mechanism Insights. Polymers. 2023; 15(5):1157. https://doi.org/10.3390/polym15051157
Chicago/Turabian StyleAl-Ghamdi, Ahmed A., Ahmed A. Galhoum, Ahmed Alshahrie, Yusuf A. Al-Turki, Amal M. Al-Amri, and S. Wageh. 2023. "Superparamagnetic Multifunctionalized Chitosan Nanohybrids for Efficient Copper Adsorption: Comparative Performance, Stability, and Mechanism Insights" Polymers 15, no. 5: 1157. https://doi.org/10.3390/polym15051157