Boosting the Antibacterial Performance of Natural Rubber Latex Foam by Introducing Silver-Doped Zinc Oxide
Abstract
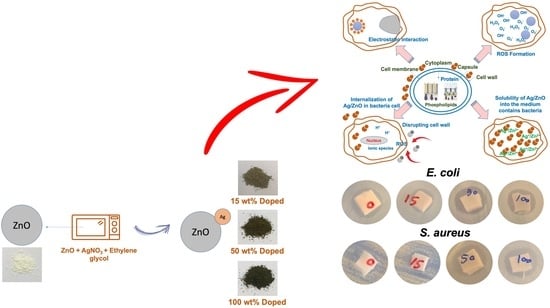
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Synthesis of ZnO
2.3. Preparation of Ag-Doped ZnO
2.4. Characterization of Ag-Doped ZnO
2.5. Preparation of NR Latex Foam Filled with ZnO and Ag-Doped ZnO
2.6. Measurement of Physical Properties
2.7. Antibacterial Study
3. Results and Discussion
3.1. Characterization of ZnO and Ag-Doped ZnO
3.2. Morphology, Elemental Analysis and Functionalities
3.3. Antibacterial Performance
3.4. Physical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sethulekshmi, A.S.; Saritha, A.; Joseph, K. A comprehensive review on the recent advancements in natural rubber nanocomposites. Int. J. Biol. Macromol. 2022, 194, 819–842. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Z.; Huang, Y.; Zhao, H.; Chen, Z.; Gao, K.; Yue, T.; Zhang, L.; Liu, J. Structure-Mechanics Relation of Natural Rubber: Insights from Molecular Dynamics Simulations. ACS Appl. Polym. Mater. 2022, 4, 3575–3586. [Google Scholar] [CrossRef]
- Cesar, M.B.; Borges, F.A.; Bilck, A.P.; Yamashita, F.; Paulino, C.G.; Herculano, R.D. Development and Characterization of Natural Rubber Latex and Polylactic Acid Membranes for Biomedical Application. J. Polym. Environ. 2020, 28, 220–230. [Google Scholar] [CrossRef]
- Borges, F.A.; Filho, E.d.A.; Miranda, M.C.R.; Santos, M.L.d.; Herculano, R.D.; Guastaldi, A.C. Natural rubber latex coated with calcium phosphate for biomedical application. J. Biomater. Sci. Polym. Ed. 2015, 26, 1256–1268. [Google Scholar] [CrossRef]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnol. 2016, 14, 73. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of Photogenerated Reactive Oxygen Species and Correlation with the Antibacterial Properties of Engineered Metal-Oxide Nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef]
- Shuai, C.; Xu, Y.; Feng, P.; Wang, G.; Xiong, S.; Peng, S. Antibacterial polymer scaffold based on mesoporous bioactive glass loaded with in situ grown silver. Chem. Eng. J. 2019, 374, 304–315. [Google Scholar] [CrossRef]
- Kumar, V.; Jolivalt, C.; Pulpytel, J.; Jafari, R.; Arefi-Khonsari, F. Development of silver nanoparticle loaded antibacterial polymer mesh using plasma polymerization process. J. Biomed. Mater. Res. Part A 2013, 101A, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Yeo, S.Y.; Yi, S.C. The effect of filler particle size on the antibacterial properties of compounded polymer/silver fibers. J. Mater. Sci. 2005, 40, 5407–5411. [Google Scholar] [CrossRef]
- Georgekutty, R.; Seery, M.K.; Pillai, S.C. A Highly Efficient Ag-ZnO Photocatalyst: Synthesis, Properties, and Mechanism. J. Phys. Chem. C 2008, 112, 13563–13570. [Google Scholar] [CrossRef] [Green Version]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murth, H.C.A. A Review on Enhancing the Antibacterial Activity of ZnO: Mechanisms and Microscopic Investigation. Nanoscale Res. Lett. 2020, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Sarih, N.M.; Gwee, K.; Maher, S.; Rashid, A.A. Natural Rubber (NR) Latex Films with Antimicrobial Properties for Stethoscope Diaphragm Covers. Materials 2022, 15, 3433. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Su, Y.; Wang, D.; Mao, Y.; Wang, W.; Liu, L.; Wen, S. High antibacterial and barrier properties of natural rubber comprising of silver-loaded graphene oxide. Int. J. Biol. Macromol. 2022, 195, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-H.; Li, J.-H.; Kumar, A.; Li, G.; Yang, Y. Doping of the Metal Oxide Nanostructure and its Influence in Organic Electronics. Adv. Funct. Mater. 2009, 19, 1241–1246. [Google Scholar] [CrossRef]
- Ogale, S.B. Dilute Doping, Defects, and Ferromagnetism in Metal Oxide Systems. Adv. Mater. 2010, 22, 3125–3155. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, C.; Zheng, B.; Geng, X.; Debliquy, M. Hydrogen sensors based on noble metal doped metal-oxide semiconductor: A review. Int. J. Hydrogen Energy 2017, 42, 20386–20397. [Google Scholar] [CrossRef]
- Hämäläinen, J.; Ritala, M.; Leskelä, M. Atomic Layer Deposition of Noble Metals and Their Oxides. Chem. Mater. 2014, 26, 786–801. [Google Scholar] [CrossRef]
- Mageshwari, K.; Nataraj, D.; Pal, T.; Sathyamoorthy, R.; Park, J. Improved photocatalytic activity of ZnO coupled CuO nanocomposites synthesized by reflux condensation method. J. Alloys Compd. 2015, 625, 362–370. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, D.; Song, Z.; Shan, C.; Zhang, Z.; Li, B.; Shen, D. Gold nanoparticles modified ZnO nanorods with improved photocatalytic activity. J. Colloid Interface Sci. 2011, 363, 175–181. [Google Scholar] [CrossRef]
- Etacheri, V.; Roshan, R.; Kumar, V. Mg-Doped ZnO Nanoparticles for Efficient Sunlight-Driven Photocatalysis. ACS Appl. Mater. Interfaces 2012, 4, 2717–2725. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xin, J.H.; Yang, Y.; Liu, H.; Xu, L.; Hu, J. The characteristics and photocatalytic activities of silver doped ZnO nanocrystallites. Appl. Surf. Sci. 2004, 227, 312–317. [Google Scholar] [CrossRef]
- Talari, M.K.; Majeed, A.B.D.; Tripathi, D.K.; Tripathy, M. Synthesis, characterization and antimicrobial investigation of mechanochemically processed silver doped ZnO nanoparticles. Chem. Pharm. Bull 2012, 60, 818–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendran, R.; Mani, A. Photocatalytic, antibacterial and anticancer activity of silver-doped zinc oxide nanoparticles. J. Saudi Chem. Soc. 2020, 24, 1010–1024. [Google Scholar] [CrossRef]
- Masa, A.; Jehsoh, N.; Saiwari, S.; Dueramae, S.; Hayeemasae, N. Microwave-assisted silver-doped zinc oxide towards antibacterial and mechanical performances of natural rubber latex film. Mater. Today Commun. 2023, 34, 105475. [Google Scholar] [CrossRef]
- ASTM D575; Standard Test Methods for Rubber Properties in Compression. American Standard of Testing of Materials: West Conshohocken, PA, USA, 2018.
- ASTM D395; Standard Test Methods for Rubber Property—Compression Set. American Standard of Testing of Materials: West Conshohocken, PA, USA, 2018.
- Liu, H.; Hu, Y.; Zhang, Z.; Liu, X.; Jia, H.; Xu, B. Synthesis of spherical Ag/ZnO heterostructural composites with excellent photocatalytic activity under visible light and UV irradiation. Appl. Surf. Sci. 2015, 355, 644–652. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Wongwiwat, N.; Thongtem, T.; Thongtem, S. Microwave-assisted solution synthesis and photocatalytic activity of Ag nanoparticles supported on ZnO nanostructure flowers. Res. Chem. Intermed. 2018, 44, 7427–7436. [Google Scholar] [CrossRef]
- Srivastava, V.; Gusain, D.; Sharma, Y.C. Synthesis, characterization and application of zinc oxide nanoparticles (n-ZnO). Ceram. Int. 2013, 39, 9803–9808. [Google Scholar] [CrossRef]
- Sangeetha, G.; Rajeshwari, S.; Venckatesh, R. Green synthesis of zinc oxide nanoparticles by aloe barbadensis miller leaf extract: Structure and optical properties. Mater. Res. Bull. 2011, 46, 2560–2566. [Google Scholar] [CrossRef]
- Nabil, H.; Ismail, H.; Azura, A.R. Comparison of thermo-oxidative ageing and thermal analysis of carbon black-filled NR/Virgin EPDM and NR/Recycled EPDM blends. Polym. Test. 2013, 32, 631–639. [Google Scholar] [CrossRef]
- Ramesan, M.T.; Siji, C.; Kalaprasad, G.; Bahuleyan, B.K.; Al-Maghrabi, M.A. Effect of Silver Doped Zinc Oxide as Nanofiller for the Development of Biopolymer Nanocomposites from Chitin and Cashew Gum. J. Polym. Environ. 2018, 26, 2983–2991. [Google Scholar] [CrossRef]
- Li, Y.; Liao, C.; Tjong, S.C. Recent Advances in Zinc Oxide Nanostructures with Antimicrobial Activities. Int. J. Mol. Sci. 2020, 21, 8836. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Immobilized silver nanoparticles enhance contact killing and show highest efficacy: Elucidation of the mechanism of bactericidal action of silver. Nanoscale 2013, 5, 7328. [Google Scholar] [CrossRef] [PubMed] [Green Version]














| Ingredients | Quantity (phr) |
|---|---|
| 60% HA | 100 |
| 20% KOL | 2.0 |
| 50% ZDEC | 1.0 |
| 50% ZMBT | 1.0 |
| 50% Sulfur | 2.5 |
| 50% Wingstay L | 1.5 |
| 15% DPG | 1.2 |
| 25% ZnO or Ag-doped ZnO | 2.0 |
| 20% SSF | 1.2 |
| AgNO3 Content (wt%) | Particle Size (nm) | |
|---|---|---|
| Ag | ZnO | |
| 15 | 47 ± 6 | 305 ± 40 |
| 50 | 45 ± 4 | 310 ± 40 |
| 100 | 46 ± 5 | 300 ± 40 |
| Comparison for Rubber Formulations | E. coli | S. aureus | ||
|---|---|---|---|---|
| p-Value | p < 0.05 | p-Value | p < 0.05 | |
| ZnO vs. 15 wt% | 0.413 | No | 1 | No |
| ZnO vs. 50 wt% | <0.001 | Yes | <0.001 | Yes |
| ZnO vs. 100 wt% | <0.001 | Yes | <0.001 | Yes |
| Comparison for Rubber Formulations | E. coli | S. aureus | ||
| p-Value | p < 0.05 | p-Value | p < 0.05 | |
| ZnO vs. 15 wt% | <0.001 | Yes | <0.001 | Yes |
| ZnO vs. 50 wt% | <0.001 | Yes | <0.001 | Yes |
| ZnO vs. 100 wt% | <0.001 | Yes | <0.001 | Yes |
| Source of Variation | E. coli | S. aureus | ||
| p-Value | p < 0.05 | p-Value | p < 0.05 | |
| Formulations | <0.001 | Yes | <0.001 | Yes |
| Contact times | 0.061 | No | 0.084 | No |
| AgNO3 Content (wt%) | Density (g/cm3) | Compression Set (%) | Stress at 25% Compression (kPa) |
|---|---|---|---|
| 0 | 0.15 ± 0.01 | 14.75 ± 0.61 | 9.85 ± 0.18 |
| 15 | 0.15 ± 0.01 | 14.75 ± 0.80 | 10.00 ± 0.25 |
| 50 | 0.15 ± 0.01 | 14.80 ± 0.91 | 9.95 ± 0.33 |
| 100 | 0.16 ± 0.01 | 14.75 ± 0.81 | 10.50 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masa, A.; Jehsoh, N.; Dueramae, S.; Hayeemasae, N. Boosting the Antibacterial Performance of Natural Rubber Latex Foam by Introducing Silver-Doped Zinc Oxide. Polymers 2023, 15, 1040. https://doi.org/10.3390/polym15041040
Masa A, Jehsoh N, Dueramae S, Hayeemasae N. Boosting the Antibacterial Performance of Natural Rubber Latex Foam by Introducing Silver-Doped Zinc Oxide. Polymers. 2023; 15(4):1040. https://doi.org/10.3390/polym15041040
Chicago/Turabian StyleMasa, Abdulhakim, Nureeyah Jehsoh, Sawitree Dueramae, and Nabil Hayeemasae. 2023. "Boosting the Antibacterial Performance of Natural Rubber Latex Foam by Introducing Silver-Doped Zinc Oxide" Polymers 15, no. 4: 1040. https://doi.org/10.3390/polym15041040