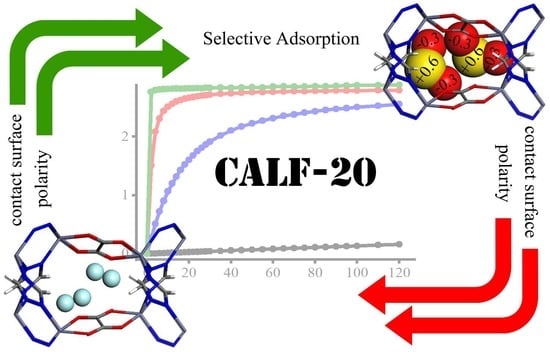
Exploring the Potential of a Highly Scalable Metal-Organic Framework CALF-20 for Selective Gas Adsorption at Low Pressure
Abstract
:1. Introduction
2. Computational Details
2.1. Model Construction
2.2. GCMC Simulation
2.3. MD Simulation
3. Results and Discussion
3.1. Adsorption Isotherm
3.2. Radial Distribution Function (RDF)
3.3. Simulation Snapshot Analysis
3.4. Interaction Energies
3.5. Henry Coefficient (KH) and Isosteric Heat of Adsorption (Qst)
3.6. Mean Square Displacement (MSD)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, J.; Xie, L.-H.; Li, J.-R.; Ma, Y.; Seminario, J.M.; Balbuena, P.B. CO2 capture and separations using MOFs: Computational and experimental studies. Chem. Rev. 2017, 117, 9674–9754. [Google Scholar] [CrossRef]
- Wang, T.C.; Bury, W.; Gómez-Gualdrón, D.A.; Vermeulen, N.A.; Mondloch, J.E.; Deria, P.; Zhang, K.; Moghadam, P.Z.; Sarjeant, A.A.; Snurr, R.Q. Ultrahigh surface area zirconium MOFs and insights into the applicability of the BET theory. J. Am. Chem. Soc. 2015, 137, 3585–3591. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, M.; Chen, C.; Zhang, Y.; Gu, Y.; Wang, Q.; Zhang, W.; Pan, Y.; Ma, J.; Bai, J. A low symmetry cluster meets a low symmetry ligand to sharply boost MOF thermal stability. Chem. Commun. 2020, 56, 11985–11988. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, Y.; Wang, J.; Xu, Y.; Bian, L.; Ju, Q.; Wang, Y.; Fang, Z. Defects engineering simultaneously enhances activity and recyclability of MOFs in selective hydrogenation of biomass. Nat. Commun. 2022, 13, 2068. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, S.M.; Nandy, A.; Jablonka, K.M.; Ongari, D.; Janet, J.P.; Boyd, P.G.; Lee, Y.; Smit, B.; Kulik, H.J. Understanding the diversity of the metal-organic framework ecosystem. Nat. Commun. 2020, 11, 4068. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, X.; Thang, A.Q.; Li, F.; Chen, D.; Geng, H.; Rui, X.; Yan, Q. Vanadium-based metal-organic frameworks and their derivatives for electrochemical energy conversion and storage. SmartMat 2022, 3, 384–416. [Google Scholar] [CrossRef]
- Haldar, D.; Duarah, P.; Purkait, M.K. MOFs for the treatment of arsenic, fluoride and iron contaminated drinking water: A review. Chemosphere 2020, 251, 126388. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.-Q.; Jiang, H.-L.; Sun, L.-B. Metal–organic frameworks for heterogeneous basic catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef]
- Lismont, M.; Dreesen, L.; Wuttke, S. Metal-organic framework nanoparticles in photodynamic therapy: Current status and perspectives. Adv. Funct. Mater. 2017, 27, 1606314. [Google Scholar] [CrossRef]
- Lázaro, I.A.; Forgan, R.S. Application of zirconium MOFs in drug delivery and biomedicine. Coord. Chem. Rev. 2019, 380, 230–259. [Google Scholar] [CrossRef] [Green Version]
- Szczęśniak, B.; Choma, J.; Jaroniec, M. Gas adsorption properties of hybrid graphene-MOF materials. J. Colloid Interface Sci. 2018, 514, 801–813. [Google Scholar] [CrossRef]
- Petit, C. Present and future of MOF research in the field of adsorption and molecular separation. Curr. Opin. Chem. Eng. 2018, 20, 132–142. [Google Scholar] [CrossRef]
- Ghanbari, T.; Abnisa, F.; Daud, W.M.A.W. A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci. Total Environ. 2020, 707, 135090. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Luo, Z.; Li, B.; Yuan, D. Microporous metal–organic framework based on ligand-truncation strategy with high performance for gas adsorption and separation. Inorg. Chem. 2017, 56, 10215–10219. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, G.; Gu, C.; Liu, W.; Xu, J.; Li, B.; Wang, W. Rational synthesis of a novel 3,3,5-c polyhedral metal–organic framework with high thermal stability and hydrogen storage capability. J. Mater. Chem. A 2016, 4, 11630–11634. [Google Scholar] [CrossRef]
- Kong, X.-J.; Zhang, Y.-Z.; He, T.; Wu, X.-Q.; Xu, M.-M.; Wang, S.-N.; Xie, L.-H.; Li, J.-R. Two interpenetrated metal–organic frameworks with a slim ethynyl-based ligand: Designed for selective gas adsorption and structural tuning. CrystEngComm 2018, 20, 6018–6025. [Google Scholar] [CrossRef]
- Zheng, B.; Luo, X.; Wang, Z.; Zhang, S.; Yun, R.; Huang, L.; Zeng, W.; Liu, W. An unprecedented water stable acylamide-functionalized metal–organic framework for highly efficient CH4/CO2 gas storage/separation and acid–base cooperative catalytic activity. Inorg. Chem. Front. 2018, 5, 2355–2363. [Google Scholar] [CrossRef]
- Cai, Y.; Zou, L.; Ji, Q.; Yong, J.; Qian, X.; Gao, J. Two dimensional Ti-based metal-organic framework with polar oxygen atoms on the pore surface for efficient gas separation. Polyhedron 2020, 190, 114771. [Google Scholar] [CrossRef]
- Teo, W.L.; Zhou, W.; Qian, C.; Zhao, Y. Industrializing metal–organic frameworks: Scalable synthetic means and their transformation into functional materials. Mater. Today 2021, 47, 170–186. [Google Scholar] [CrossRef]
- Lin, J.-B.; Nguyen, T.T.T.; Vaidhyanathan, R.; Burner, J.; Taylor, J.M.; Durekova, H.; Akhtar, F.; Mah, R.K.; Ghaffari-Nik, O.; Marx, S. A scalable metal-organic framework as a durable physisorbent for carbon dioxide capture. Science 2021, 374, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Czaja, A.U.; Trukhan, N.; Müller, U. Industrial applications of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Julien, P.A.; Mottillo, C.; Friščić, T. Metal–organic frameworks meet scalable and sustainable synthesis. Green Chem. 2017, 19, 2729–2747. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Lin, J.-B.; Shimizu, G.K.H.; Rajendran, A. Separation of CO2 and N2 on a hydrophobic metal organic framework CALF-20. Chem. Eng. J. 2022, 442, 136263. [Google Scholar] [CrossRef]
- Barea, E.; Montoro, C.; Navarro, J.A.R. Toxic gas removal–metal–organic frameworks for the capture and degradation of toxic gases and vapours. Chem. Soc. Rev. 2014, 43, 5419–5430. [Google Scholar] [CrossRef]
- Korica, M.; Balić, I.; van Wyk, L.M.; van Heerden, D.P.; Nikolayenko, V.I.; Barbour, L.J.; Jednačak, T.; Đilović, I.; Balić, T. Inclusion of CO2, NH3, SO2, Cl2 and H2S in porous N4O4-donor macrocyclic Schiff base. Microporous Mesoporous Mater. 2022, 332, 111708. [Google Scholar] [CrossRef]
- Fuge, R. Sources of halogens in the environment, influences on human and animal health. Environ. Geochem. Health 1988, 10, 51–61. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; García-Figueroa, A.; Lavilla, I.; Bendicho, C. Nanomaterials for the detection of halides and halogen oxyanions by colorimetric and luminescent techniques: A critical overview. Trac Trends Anal. Chem. 2020, 125, 115837. [Google Scholar] [CrossRef]
- Tian, K.; Wang, X.-X.; Yu, Z.-Y.; Li, H.-Y.; Guo, X. Hierarchical and hollow Fe2O3 nanoboxes derived from metal–organic frameworks with excellent sensitivity to H2S. ACS Appl. Mater. Interfaces 2017, 9, 29669–29676. [Google Scholar] [CrossRef]
- Dong, X.; Su, Y.; Lu, T.; Zhang, L.; Wu, L.; Lv, Y. MOFs-derived dodecahedra porous Co3O4: An efficient cataluminescence sensing material for H2S. Sens. Actuators B Chem. 2018, 258, 349–357. [Google Scholar] [CrossRef]
- Yang, X.-F.; Zhu, H.-B.; Liu, M. Transition-metal-based (Zn2+ and Cd2+) metal-organic frameworks as fluorescence “turn-off” sensors for highly sensitive and selective detection of hydrogen sulfide. Inorg. Chim. Acta 2017, 466, 410–416. [Google Scholar] [CrossRef]
- Vikrant, K.; Kailasa, S.K.; Tsang, D.C.W.; Lee, S.S.; Kumar, P.; Giri, B.S.; Singh, R.S.; Kim, K.-H. Biofiltration of hydrogen sulfide: Trends and challenges. J. Clean. Prod. 2018, 187, 131–147. [Google Scholar] [CrossRef]
- Liu, D.; Quan, X.; Zhou, L.; Huang, Q.; Wang, C. Utilization of waste concrete powder with different particle size as absorbents for SO2 reduction. Constr. Build. Mater. 2021, 266, 121005. [Google Scholar] [CrossRef]
- Carter, J.H.; Han, X.; Moreau, F.Y.; Da Silva, I.; Nevin, A.; Godfrey, H.G.W.; Tang, C.C.; Yang, S.; Schröder, M. Exceptional adsorption and binding of sulfur dioxide in a robust zirconium-based metal–organic framework. J. Am. Chem. Soc. 2018, 140, 15564–15567. [Google Scholar] [CrossRef]
- Chen, X.; Shen, B.; Sun, H. Ion-exchange modified zeolites X for selective adsorption desulfurization from Claus tail gas: Experimental and computational investigations. Microporous Mesoporous Mater. 2018, 261, 227–236. [Google Scholar] [CrossRef]
- Li, J.; Kang, Y.; Li, B.; Wang, X.; Li, D. PEG-linked functionalized dicationic ionic liquids for highly efficient SO2 capture through physical absorption. Energy Fuels 2018, 32, 12703–12710. [Google Scholar] [CrossRef]
- Xing, S.; Liang, J.; Brandt, P.; Schäfer, F.; Nuhnen, A.; Heinen, T.; Boldog, I.; Möllmer, J.; Lange, M.; Weingart, O. Capture and Separation of SO2 Traces in Metal–Organic Frameworks via Pre-Synthetic Pore Environment Tailoring by Methyl Groups. Angew. Chem. Int. Ed. 2021, 60, 17998–18005. [Google Scholar] [CrossRef]
- Brandt, P.; Nuhnen, A.; Lange, M.; Möllmer, J.; Weingart, O.; Janiak, C. Metal–organic frameworks with potential application for SO2 separation and flue gas desulfurization. ACS Appl. Mater. Interfaces 2019, 11, 17350–17358. [Google Scholar] [CrossRef]
- Rodríguez-Albelo, L.M.; López-Maya, E.; Hamad, S.; Ruiz-Salvador, A.R.; Calero, S.; Navarro, J.A.R. Selective sulfur dioxide adsorption on crystal defect sites on an isoreticular metal organic framework series. Nat. Commun. 2017, 8, 14457. [Google Scholar] [CrossRef] [Green Version]
- Willems, T.F.; Rycroft, C.H.; Kazi, M.; Meza, J.C.; Haranczyk, M. Algorithms and tools for high-throughput geometry-based analysis of crystalline porous materials. Microporous Mesoporous Mater. 2012, 149, 134–141. [Google Scholar] [CrossRef]
- Dubbeldam, D.; Calero, S.; Ellis, D.E.; Snurr, R.Q. RASPA: Molecular simulation software for adsorption and diffusion in flexible nanoporous materials. Mol. Simul. 2016, 42, 81–101. [Google Scholar] [CrossRef]
- Rappé, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard III, W.A.; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Mayo, S.L.; Olafson, B.D.; Goddard, W.A. DREIDING: A generic force field for molecular simulations. J. Phys. Chem. 1990, 94, 8897–8909. [Google Scholar] [CrossRef]
- Bondi, A. van van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Boyd, P.G.; Moosavi, S.M.; Witman, M.; Smit, B. Force-field prediction of materials properties in metal-organic frameworks. J. Phys. Chem. Lett. 2017, 8, 357–363. [Google Scholar] [CrossRef]
- Bristow, J.K.; Tiana, D.; Walsh, A. Transferable force field for metal–organic frameworks from first-principles: BTW-FF. J. Chem. Theory Comput. 2014, 10, 4644–4652. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian, 09 Program; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Savage, M.; Yang, S.; Suyetin, M.; Bichoutskaia, E.; Lewis, W.; Blake, A.J.; Barnett, S.A.; Schröder, M. A Novel Bismuth-Based Metal–Organic Framework for High Volumetric Methane and Carbon Dioxide Adsorption. Chem. Eur. J. 2014, 20, 8024–8029. [Google Scholar] [CrossRef]
- Burtch, N.C.; Jasuja, H.; Dubbeldam, D.; Walton, K.S. Molecular-level insight into unusual low pressure CO2 affinity in pillared metal–organic frameworks. J. Am. Chem. Soc. 2013, 135, 7172–7180. [Google Scholar] [CrossRef]
- Brandt, P.; Xing, S.-H.; Liang, J.; Kurt, G.; Nuhnen, A.; Weingart, O.; Janiak, C. Zirconium and Aluminum MOFs for Low-Pressure SO2 Adsorption and Potential Separation: Elucidating the Effect of Small Pores and NH2 Groups. ACS Appl. Mater. Interfaces 2021, 13, 29137–29149. [Google Scholar] [CrossRef]
- Xia, X.; Hu, G.; Li, W.; Li, S. Understanding reduced CO2 uptake of ionic liquid/metal–organic framework (IL/MOF) composites. ACS Appl. Nano Mater. 2019, 2, 6022–6029. [Google Scholar] [CrossRef]
- Anderson, R.; Schweitzer, B.; Wu, T.; Carreon, M.A.; Gómez-Gualdrón, D.A. Molecular simulation insights on Xe/Kr separation in a set of nanoporous crystalline membranes. ACS Appl. Mater. Interfaces 2018, 10, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Avci, G.; Velioglu, S.; Keskin, S. High-throughput screening of MOF adsorbents and membranes for H2 purification and CO2 capture. ACS Appl. Mater. Interfaces 2018, 10, 33693–33706. [Google Scholar] [CrossRef]
- Qian, J.; Chen, G.; Xiao, S.; Li, H.; Ouyang, Y.; Wang, Q. Switching Xe/Kr adsorption selectivity in modified SBMOF-1: A theoretical study. RSC Adv. 2020, 10, 17195–17204. [Google Scholar] [CrossRef] [PubMed]
- Rogge, S.M.J.; Goeminne, R.; Demuynck, R.; Gutiérrez-Sevillano, J.J.; Vandenbrande, S.; Vanduyfhuys, L.; Waroquier, M.; Verstraelen, T.; Van Speybroeck, V. Modeling Gas Adsorption in Flexible Metal–Organic Frameworks via Hybrid Monte Carlo/Molecular Dynamics Schemes. Adv. Theory Simul. 2019, 2, 1800177. [Google Scholar] [CrossRef]
- Seehamart, K.; Nanok, T.; Kärger, J.; Chmelik, C.; Krishna, R.; Fritzsche, S. Investigating the reasons for the significant influence of lattice flexibility on self-diffusivity of ethane in Zn (tbip). Microporous Mesoporous Mater. 2010, 130, 92–96. [Google Scholar] [CrossRef]
- De Toni, M.; Jonchiere, R.; Pullumbi, P.; Coudert, F.; Fuchs, A.H. How can a hydrophobic MOF be water-unstable? Insight into the hydration mechanism of IRMOFs. ChemPhysChem 2012, 13, 3497–3503. [Google Scholar] [CrossRef]
- Polat, H.M.; Zeeshan, M.; Uzun, A.; Keskin, S. Unlocking CO2 separation performance of ionic liquid/CuBTC composites: Combining experiments with molecular simulations. Chem. Eng. J. 2019, 373, 1179–1189. [Google Scholar] [CrossRef]
- Altundal, O.F.; Altintas, C.; Keskin, S. Can COFs replace MOFs in flue gas separation? high-throughput computational screening of COFs for CO2/N2 separation. J. Mater. Chem. A 2020, 8, 14609–14623. [Google Scholar] [CrossRef]
- Luna-Triguero, A.; Sławek, A.; Sánchez-de-Armas, R.; Gutiérrez-Sevillano, J.J.; Ania, C.O.; Parra, J.B.; Vicent-Luna, J.M.; Calero, S. π-Complexation for olefin/paraffin separation using aluminosilicates. Chem. Eng. J. 2020, 380, 122482. [Google Scholar] [CrossRef]
- Altintas, C.; Avci, G.; Daglar, H.; Gulcay, E.; Erucar, I.; Keskin, S. Computer simulations of 4240 MOF membranes for H2/CH4 separations: Insights into structure–performance relations. J. Mater. Chem. A 2018, 6, 5836–5847. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhang, X.; Kang, Z.; Liu, X.; Sun, D. Isoreticular chemistry within metal–organic frameworks for gas storage and separation. Coord. Chem. Rev. 2021, 443, 213968. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Lin, R.-B.; Zhou, W.; Zhang, Z.; Xiang, S.; Chen, B. Porous metal-organic frameworks for gas storage and separation: Status and challenges. EnergyChem 2019, 1, 100006. [Google Scholar] [CrossRef]
- Xue, D.-X.; Belmabkhout, Y.; Shekhah, O.; Jiang, H.; Adil, K.; Cairns, A.J.; Eddaoudi, M. Tunable rare earth fcu-MOF platform: Access to adsorption kinetics driven gas/vapor separations via pore size contraction. J. Am. Chem. Soc. 2015, 137, 5034–5040. [Google Scholar] [CrossRef]
- Nugent, P.; Belmabkhout, Y.; Burd, S.D.; Cairns, A.J.; Luebke, R.; Forrest, K.; Pham, T.; Ma, S.; Space, B.; Wojtas, L. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 2013, 495, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Mohideen, M.I.H.; Pillai, R.S.; Adil, K.; Bhatt, P.M.; Belmabkhout, Y.; Shkurenko, A.; Maurin, G.; Eddaoudi, M. A fine-tuned MOF for gas and vapor separation: A multipurpose adsorbent for acid gas removal, dehydration, and BTX sieving. Chem 2017, 3, 822–833. [Google Scholar] [CrossRef]
- Li, K.; Olson, D.H.; Seidel, J.; Emge, T.J.; Gong, H.; Zeng, H.; Li, J. Zeolitic imidazolate frameworks for kinetic separation of propane and propene. J. Am. Chem. Soc. 2009, 131, 10368–10369. [Google Scholar] [CrossRef]
- Cai, J.; Yu, J.; Xu, H.; He, Y.; Duan, X.; Cui, Y.; Wu, C.; Chen, B.; Qian, G. A doubly interpenetrated metal–organic framework with open metal sites and suitable pore sizes for highly selective separation of small hydrocarbons at room temperature. Cryst. Growth Des. 2013, 13, 2094–2097. [Google Scholar] [CrossRef]
- Cho, K.H.; Borges, D.D.; Lee, U.; Lee, J.S.; Yoon, J.W.; Cho, S.J.; Park, J.; Lombardo, W.; Moon, D.; Sapienza, A. Rational design of a robust aluminum metal-organic framework for multi-purpose water-sorption-driven heat allocations. Nat. Commun. 2020, 11, 5112. [Google Scholar] [CrossRef]
- Hanikel, N.; Prévot, M.S.; Fathieh, F.; Kapustin, E.A.; Lyu, H.; Wang, H.; Diercks, N.J.; Glover, T.G.; Yaghi, O.M. Rapid cycling and exceptional yield in a metal-organic framework water harvester. ACS Cent. Sci. 2019, 5, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yazaydin, A.O. The effect of SO2 on CO2 capture in zeolitic imidazolate frameworks. Phys. Chem. Chem. Phys. 2013, 15, 11856–11861. [Google Scholar] [CrossRef] [PubMed]
- Canturk, B.; Kurt, A.S.; Gurdal, Y. Models used for permeability predictions of nanoporous materials revisited for H2/CH4 and H2/CO2 mixtures. Sep. Purif. Technol. 2022, 297, 121463. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, X.; Li, H.A.; Hou, J. Competitive adsorption behavior of hydrocarbon(s)/CO2 mixtures in a double-nanopore system using molecular simulations. Fuel 2019, 252, 612–621. [Google Scholar] [CrossRef]
- Li, L.; Da Silva, I.; Kolokolov, D.I.; Han, X.; Li, J.; Smith, G.; Cheng, Y.; Daemen, L.L.; Morris, C.G.; Godfrey, H.G.W. Post-synthetic modulation of the charge distribution in a metal–organic framework for optimal binding of carbon dioxide and sulfur dioxide. Chem. Sci. 2019, 10, 1472–1482. [Google Scholar] [CrossRef]
- Borzehandani, M.Y.; Abdulmalek, E.; Abdul Rahman, M.B.; Latif, M.A.M. Elucidating the Aromatic Properties of Covalent Organic Frameworks Surface for Enhanced Polar Solvent Adsorption. Polymers 2021, 13, 1861. [Google Scholar] [CrossRef] [PubMed]
- Kareem, F.A.A.; Shariff, A.M.; Ullah, S.; Mellon, N.; Keong, L.K. Adsorption of pure and predicted binary (CO2:CH4) mixtures on 13X-Zeolite: Equilibrium and kinetic properties at offshore conditions. Microporous Mesoporous Mater. 2018, 267, 221–234. [Google Scholar] [CrossRef]
- Hefti, M.; Marx, D.; Joss, L.; Mazzotti, M. Adsorption equilibrium of binary mixtures of carbon dioxide and nitrogen on zeolites ZSM-5 and 13X. Microporous Mesoporous Mater. 2015, 215, 215–228. [Google Scholar] [CrossRef]
- Saha, D.; Bao, Z.; Jia, F.; Deng, S. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A. Environ. Sci. Technol. 2010, 44, 1820–1826. [Google Scholar] [CrossRef]
- Moghadam, P.Z.; Fairen-Jimenez, D.; Snurr, R.Q. Efficient identification of hydrophobic MOFs: Application in the capture of toxic industrial chemicals. J. Mater. Chem. A 2016, 4, 529–536. [Google Scholar] [CrossRef]
- Wu, X.; Shahrak, M.N.; Yuan, B.; Deng, S. Synthesis and characterization of zeolitic imidazolate framework ZIF-7 for CO2 and CH4 separation. Microporous Mesoporous Mater. 2014, 190, 189–196. [Google Scholar] [CrossRef]
- Zacharia, R.; Gomez, L.F.; Chahine, R.; Cossement, D.; Benard, P. Thermodynamics and kinetics of CH4/CO2 binary mixture separation by metal-organic frameworks from isotope exchange and adsorption break-through. Microporous Mesoporous Mater. 2018, 263, 165–172. [Google Scholar] [CrossRef]
- Martínez-Ahumada, E.; He, D.; Berryman, V.; López-Olvera, A.; Hernandez, M.; Jancik, V.; Martis, V.; Vera, M.A.; Lima, E.; Parker, D.J. SO2 capture using porous organic cages. Angew. Chem. Int. Ed. 2021, 60, 17556–17563. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.; Venkataramanan, N.S. Sustainable metallocavitand for flue gas-selective sorption: A multiscale study. J. Phys. Chem. C 2019, 123, 3188–3202. [Google Scholar] [CrossRef]
- Furukawa, H.; Yaghi, O.M. Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, X.; Wang, W.; Cao, D. Selective capture of trace sulfur gas by porous covalent-organic materials. Chem. Eng. Sci. 2015, 135, 373–380. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhang, C.; Li, Z.; Zhu, J.; Lu, B. Molecular simulation of gases competitive adsorption in lignite and analysis of original CO desorption. Sci. Rep. 2021, 11, 11706. [Google Scholar] [CrossRef] [PubMed]
- Ramanayaka, S.; Vithanage, M.; Sarmah, A.; An, T.; Kim, K.-H.; Ok, Y.S. Performance of metal–organic frameworks for the adsorptive removal of potentially toxic elements in a water system: A critical review. RSC Adv. 2019, 9, 34359–34376. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Maiti, A. Adsorption and decomposition of H2S on MgO (100), NiMgO (100), and ZnO (0001) surfaces: A first-principles density functional study. J. Phys. Chem. B 2000, 104, 3630–3638. [Google Scholar] [CrossRef]
- Britt, D.; Tranchemontagne, D.; Yaghi, O.M. Metal-organic frameworks with high capacity and selectivity for harmful gases. Proc. Natl. Acad. Sci. USA 2008, 105, 11623–11627. [Google Scholar] [CrossRef]
- Tulchinsky, Y.; Hendon, C.H.; Lomachenko, K.A.; Borfecchia, E.; Melot, B.C.; Hudson, M.R.; Tarver, J.D.; Korzyński, M.D.; Stubbs, A.W.; Kagan, J.J. Reversible capture and release of Cl2 and Br2 with a redox-active metal–organic framework. J. Am. Chem. Soc. 2017, 139, 5992–5997. [Google Scholar] [CrossRef] [Green Version]
- Tranchemontagne, D.J.; Hunt, J.R.; Yaghi, O.M. Room temperature synthesis of metal-organic frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 2008, 64, 8553–8557. [Google Scholar] [CrossRef]
- Lai, L.S.; Yeong, Y.F.; Ani, N.C.; Lau, K.K.; Shariff, A.M. Effect of synthesis parameters on the formation of zeolitic imidazolate framework 8 (ZIF-8) nanoparticles for CO2 adsorption. Part. Sci. Technol. 2014, 32, 520–528. [Google Scholar] [CrossRef]
- Åhlén, M.; Jaworski, A.; Strømme, M.; Cheung, O. Selective adsorption of CO2 and SF6 on mixed-linker ZIF-7–8s: The effect of linker substitution on uptake capacity and kinetics. Chem. Eng. J. 2021, 422, 130117. [Google Scholar] [CrossRef]
- Lgaz, H.; Lee, H. Computational investigation on interaction mechanism of sulfur mustard adsorption by zeolitic imidazolate frameworks ZIF-8 and ZIF-67: Insights from periodic and cluster DFT calculations. J. Mol. Liq. 2021, 344, 117705. [Google Scholar] [CrossRef]
- Amirjalayer, S.; Tafipolsky, M.; Schmid, R. Molecular dynamics simulation of benzene diffusion in mof-5: Importance of lattice dynamics. Angew. Chem. Int. Ed. 2007, 46, 463–466. [Google Scholar] [CrossRef]
- Farzi, N.; Salehi, N.; Mahboubi, A. Molecular dynamics simulation of acetylene diffusion in MOF-508a and MOF-508b. Microporous Mesoporous Mater. 2017, 248, 246–255. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Oxford University Press: Oxford, UK, 2017; ISBN 0192524704. [Google Scholar]
- Mongalo, L.; Lopis, A.S.; Venter, G.A. Molecular dynamics simulations of the structural properties and electrical conductivities of CaO–MgO–Al2O3–SiO2 melts. J. Non. Cryst. Solids 2016, 452, 194–202. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, G.; Sun, Y.; Huang, L. A computational study of water in UiO-66 Zr-MOFs: Diffusion, hydrogen bonding network, and confinement effect. AIChE J. 2021, 67, e17035. [Google Scholar] [CrossRef]







| Gas | KH CALF-20 | Qst PPX 1 | Qst COF-10 2 | Qst CALF-20 3 |
|---|---|---|---|---|
| F2 | 0.001 | 11.72 | - | 16.35 |
| Cl2 | 1.018 | 35.15 | - | 41.84 |
| H2S | 0.139 | 28.45 | 15.75 | 31.88 |
| SO2 | 22.743 | - | 17.68 | 45.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borzehandani, M.Y.; Jorabchi, M.N.; Abdulmalek, E.; Abdul Rahman, M.B.; Mohammad Latif, M.A. Exploring the Potential of a Highly Scalable Metal-Organic Framework CALF-20 for Selective Gas Adsorption at Low Pressure. Polymers 2023, 15, 760. https://doi.org/10.3390/polym15030760
Borzehandani MY, Jorabchi MN, Abdulmalek E, Abdul Rahman MB, Mohammad Latif MA. Exploring the Potential of a Highly Scalable Metal-Organic Framework CALF-20 for Selective Gas Adsorption at Low Pressure. Polymers. 2023; 15(3):760. https://doi.org/10.3390/polym15030760
Chicago/Turabian StyleBorzehandani, Mostafa Yousefzadeh, Majid Namayandeh Jorabchi, Emilia Abdulmalek, Mohd Basyaruddin Abdul Rahman, and Muhammad Alif Mohammad Latif. 2023. "Exploring the Potential of a Highly Scalable Metal-Organic Framework CALF-20 for Selective Gas Adsorption at Low Pressure" Polymers 15, no. 3: 760. https://doi.org/10.3390/polym15030760