Removal of Bromine from Polymer Blends with a Composition Simulating That Found in Waste Electric and Electronic Equipment through a Facile and Environmentally Friendly Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blends Preparation
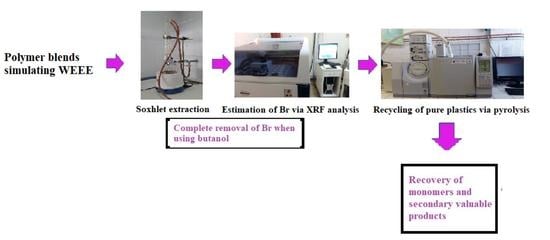
2.3. Soxhlet Extraction Procedure
2.4. Analytical Methods
3. Results and Discussion
3.1. Effect of Solvent Extraction on the Polymer Structure in the Blends
3.2. Bromine Content before and after Soxhlet Extraction—XRF Results
3.3. Pyrolysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Achilias, D.; Andriotis, L.; Koutsidis, I.A.; Louka, D.A.; Nianias, N.P.; Siafaka, P.; Tsagkalias, I.; Tsintzou, G. Recent advances in the chemical recycling of polymers (PP, PS, LDPE, HDPE, PVC, PC, nylon, PMMA). In Material Recycling—Trends and Perspectives; Achilias, D., Ed.; InTech Open: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Yu, J.; Wang, B.; Song, Z.; Xiang, J.; Hu, S.; Su, S.; Sun, L. Chemical recycling of brominated flame retarded plastics from e-waste for clean fuels production: A review. Renew. Sust. Energ Rev. 2016, 61, 433–450. [Google Scholar] [CrossRef]
- Buekens, A.; Yang, J. Recycling of WEEE plastics: A review. J. Mater. Cycles Waste Manag. 2014, 16, 415–434. [Google Scholar] [CrossRef]
- Bill, A.; Haarman, A.; Gasser, M.; Böni, H.; Rösslein, M.; Wäger, P.A. Characterizing plastics from large household appliances: Brominated flame retardants, other additives and density profiles. Res. Conserv. Recycl. 2022, 177, 105956. [Google Scholar] [CrossRef]
- Zhang, M.; Buekens, A.; Li, X. Brominated flame retardants and the formation of dioxins and furans in fires and combustion. J. Hazard. Mater. 2016, 304, 26–39. [Google Scholar] [CrossRef]
- Ilankoon, I.M.S.K.; Ghorbani, Y.; Nan Chong, M.; Herath, G.; Moyo, T.; Petersen, J. E-waste in the international context–A review of trade flows, regulations, hazards, waste management strategies and technologies for value recovery. Waste Manag. 2018, 82, 258–275. [Google Scholar] [CrossRef]
- Ma, Y.; Stubbings, W.A.; Abdallah, M.A.-E.; Cline-Cole, R.; Harrad, S. Formal waste treatment facilities as a source of halogenated flame retardants and organophosphate esters to the environment: A critical review with particular focus on outdoor air and soil. Sci. Total Environm. 2022, 807, 150747. [Google Scholar] [CrossRef]
- Chaine, C.; Hursthouse, A.S.; McLean, B.; McLellan, I.; McMahon, B.; McNulty, J.; Miller, J.; Viza, E. Recycling Plastics from WEEE: A Review of the Environmental and Human Health Challenges Associated with Brominated Flame Retardants. Intern. J. Environm. Res. Public Health 2022, 19, 766. [Google Scholar] [CrossRef]
- Nnorom, I.C.; Osibanjo, O. Sound management of brominated flame retarded (BFR) plastics from electronic wastes: State of the art and options in Nigeria. Resour. Conserv. Recycl. 2008, 52, 1362–1372. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Antonakou, E.V.; Redhwi, H.H.; Achilias, D.S. Kinetic analysis of thermal and catalytic degradation of polymers found in waste electric and electronic equipment. Thermochim. Acta 2019, 675, 69–76. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Vinu, R.; Ojha, D.K.; Nair, V. Polymer pyrolysis for resource recovery. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Reedijk, J., Ed.; Elsevier Inc.: Waltham, MA, USA, 2016. [Google Scholar] [CrossRef]
- Esposito, L.; Cafiero, L.; De Angelis, D.; Tuffi, R.; Vecchio Ciprioti, S. Valorization of the plastic residue from a WEEE treatment plant by pyrolysis. Waste Manag. 2020, 112, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Beccagutti, B.; Cafiero, L.; Pietrantonio, M.; Pucciarmati, S.; Tuffi, R.; Vecchio Ciprioti, S. Characterization of Some Real Mixed Plastics from WEEE: A Focus on Chlorine and Bromine Determination by Different Analytical Methods. Sustainability 2016, 8, 1107. [Google Scholar] [CrossRef] [Green Version]
- Charitopoulou, M.A.; Kalogiannis, K.G.; Lappas, A.A.; Achilias, D.S. Novel trends in the thermo-chemical recycling of plastics from WEEE containing brominated flame retardants. Environ. Sci. Pollut. Res. 2021, 28, 59190–59213. [Google Scholar] [CrossRef] [PubMed]
- Strobl, L.; Diefenhardt, T.; Schlummer, M.; Leege, T.; Wagner, S. Recycling potential for non-valorized plastic fractions from electrical and electronic waste. Recycling 2021, 6, 33. [Google Scholar] [CrossRef]
- Ma, C.; Yan, Q.; Yu, J.; Chen, T.; Wang, D.; Liu, S.; Bikane, K.; Sun, L. The behavior of heteroatom compounds during the pyrolysis of waste computer casing plastic under various heating conditions. J. Clean. Prod. 2019, 219, 461–470. [Google Scholar] [CrossRef]
- Bhaskar, T.; Hall, W.J.; Mitan, N.M.M.; Muto, A.; Williams, P.T.; Sakata, Y. Controlled pyrolysis of polyethylene/polypropylene/polystyrene mixed plastics with high impact polystyrene containing flame retardant: Effect of decabromo diphenylethane (DDE). Polym. Degrad. Stabil. 2007, 92, 211–221. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, S.; Yang, F.; Li, S.; Wu, J.; Liu, J.; Zhong, S.; Zeng, J. The effects of activated Al2O3 on the recycling of light oil from the catalytic pyrolysis of waste printed circuit boards. Process Safety Environm. Protect. 2015, 98, 276–284. [Google Scholar] [CrossRef]
- Wu, H.; Shen, Y.; Harada, N.; An, Q.; Yoshikawa, K. Production of Pyrolysis Oil with Low Bromine and Antimony Contents from Plastic Material Containing Brominated Flame Retardants and Antimony Trioxide. Energy Environm. Res. 2014, 4, 105–118. [Google Scholar] [CrossRef]
- Charitopoulou, M.A.; Stefanidis, S.D.; Lappas, A.A.; Achilias, D.S. Catalytic pyrolysis of polymers with brominated flame-retardants originating in waste electric and electronic equipment (WEEE) using various catalysts. Sustain. Chem. Pharm. 2022, 26, 100612. [Google Scholar] [CrossRef]
- Zhang, C.-C.; Zhang, F.-S. Removal of brominated flame retardant from electrical and electronic waste plastic by solvothermal technique. J. Hazard. Mater. 2012, 221– 222, 193–198. [Google Scholar] [CrossRef]
- Evangelopoulos, P.; Arato, S.; Persson, H.; Kantarelis, E.; Yang, W. Reduction of brominated flame retardants (BFRs) in plastics from waste electrical and electronic equipment (WEEE) by solvent extraction and the influence on their thermal decomposition. Waste Manag. 2019, 94, 165–171. [Google Scholar] [CrossRef]
- Gripon, L.; Belyamani, I.; Legros, B.; Seaudeau-Pirouley, K.; Lafranche, E.; Cauret, L. Brominated flame retardants extraction from waste electrical and electronic equipment-derived ABS using supercritical carbon dioxide. Waste Manag. 2021, 131, 313–322. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Camargo, A.D.; Bueno, M.; Parada-Alfonso, F.; Cifuentes, A.; Ibanez, E. Hansen solubility parameters for selection of green extraction solvents. Trends Anal. Chem. 2019, 118, 227–237. [Google Scholar] [CrossRef]
- Zhenova, A. Challenges in the development of new green solvents for polymer dissolution. Polym. Int. 2020, 69, 895–901. [Google Scholar] [CrossRef]
- CHEM21. Available online: http://www.chem21.eu/project/chem21-solvent-selection-guide/ (accessed on 20 November 2022).
- Altwaiq, A.M.; Wolf, M.; Eldik, R.V. Extraction of brominated flame retardants from polymeric waste material using different solvents and supercritical carbon dioxide. Anal. Chim. Acta 2003, 491, 111–123. [Google Scholar] [CrossRef]
- Zhong, Y.; Peng, P.; Huang, W. Transformation of tetrabromobisphenol A in the presence of different solvents and metals. Chemosphere 2012, 87, 1141–1148. [Google Scholar] [CrossRef]
- Yang, X.; Sun, L.; Xiang, J.; Hu, S.; Su, S. Pyrolysis and dehalogenation of plastics from waste electrical and electronic equipment (WEEE): A review. Waste Manag. 2013, 33, 462–473. [Google Scholar] [CrossRef]
- Charitopoulou, M.A.; Papadopoulou, L.; Achilias, D.S. Effect of brominated flame retardant on the pyrolysis products of polymers originating in WEEE. Environ. Sci. Pollut. Res. 2022, 29, 29570–29582. [Google Scholar] [CrossRef]
- Charitopoulou, M.A.; Papadopoulou, L.; Achilias, D.S. Microwave-assisted extraction as an effective method for the debromination of brominated flame retarded polymeric blends with a composition that simulates the plastic part of waste electric and electronic equipment (WEEE). Sustain. Chem. Pharm. 2022, 29, 100790. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters. A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Hall, W.J.; Miskolczi, N.; Onwudili, J.; Williams, P.T. Thermal processing of toxic flame-retarded polymers using a waste fluidized catalytic cracker (FCC) catalyst. Energy Fuels 2008, 22, 1691–1697. [Google Scholar] [CrossRef]











| Extraction Time (h) | Blend Name | wt.% Br Measured | % Br Reduction |
|---|---|---|---|
| 3 | Blend I | 4.613 | 17 |
| Blend II | 4.493 | 25 | |
| 6 | Blend I | 3.67 | 34 |
| Blend II | 3.243 | 46 | |
| 12 | Blend I | 1.23 | 78 |
| Blend II | 0.661 | 89 |
| Extractive Solvent | Blend Name | wt.% Br Measured | % Br Reduction |
|---|---|---|---|
| Isopropanol | Blend I | 3.67 | 34 |
| Blend II | 3.243 | 46 | |
| Ethanol | Blend I | 3.646 | 34 |
| Blend II | 3.494 | 42 | |
| Butanol | Blend I | BDL * | 100 |
| Blend II | 0.0986 | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charitopoulou, M.A.; Papadopoulou, L.; Achilias, D.S. Removal of Bromine from Polymer Blends with a Composition Simulating That Found in Waste Electric and Electronic Equipment through a Facile and Environmentally Friendly Method. Polymers 2023, 15, 709. https://doi.org/10.3390/polym15030709
Charitopoulou MA, Papadopoulou L, Achilias DS. Removal of Bromine from Polymer Blends with a Composition Simulating That Found in Waste Electric and Electronic Equipment through a Facile and Environmentally Friendly Method. Polymers. 2023; 15(3):709. https://doi.org/10.3390/polym15030709
Chicago/Turabian StyleCharitopoulou, Maria Anna, Lambrini Papadopoulou, and Dimitris S. Achilias. 2023. "Removal of Bromine from Polymer Blends with a Composition Simulating That Found in Waste Electric and Electronic Equipment through a Facile and Environmentally Friendly Method" Polymers 15, no. 3: 709. https://doi.org/10.3390/polym15030709