Extraction and Characterization of Cellulose from Agricultural By-Products of Chiang Rai Province, Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
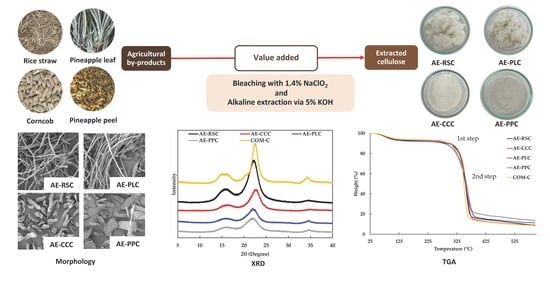
2.2. Preparation and Extraction of Cellulose from Agricultural By-Products
2.3. Physico-Chemical Composition Analysis of Cellulose from Agricultural Waste
2.4. Scanning Electron Microscopy (SEM) of Cellulose
2.5. Fourier Transform Infrared (FTIR)
2.6. X-ray Diffraction (XRD)
2.7. Thermogravimetric Analysis (TGA) of Cellulose
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Composition of Cellulose Extracted without and with Alkaline Treatment from Agricultural By-Products
3.2. Microstructure of Cellulose Extracted from Agricultural By-Product Using Alkaline Process
3.3. Fourier Transform Infrared (FTIR) of Cellulose Samples Extracted without and with Alkaline Process
3.4. X-ray Diffraction (XRD) of Cellulose Samples Extracted without and with Alkaline Process
3.5. Thermogravimetric Analysis (TGA) and Derivative Thermogravimetry (DTG) Curves of Extracted Cellulose with Alkaline Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatterjee, S.; Sharma, S. Microplastics in our oceans and marine health. Field Actions Sci. Rep. J. Field Actions 2019, 19, 54–61. [Google Scholar]
- Shiekh, K.A.; Ngiwngam, K.; Tongdeesoontorn, W. Polysaccharide-based active coatings incorporated with bioactive compounds for reducing postharvest losses of fresh fruits. Coatings 2021, 12, 8. [Google Scholar] [CrossRef]
- Thailand Office of Agricultural Economics. Thailand Foreign Agricultural Trade Statistics 2021. Available online: http://www.oae.go.th (accessed on 14 January 2022).
- Arunrat, N.; Pumijumnong, N.; Sereenonchai, S. Air-pollutant emissions from agricultural burning in Mae Chaem Basin, Chiang Mai province, Thailand. Atmosphere 2018, 9, 145. [Google Scholar] [CrossRef] [Green Version]
- Le, D.; Konsue, N. Mass transfer behavior during osmotic dehydration and vacuum impregnation of “Phulae” pineapple and the effects on dried fruit quality. Curr. Res. Nutr. Food Sci. J. 2021, 9, 308–319. [Google Scholar] [CrossRef]
- Techavuthiporn, C.; Boonyaritthongchai, P.; Supabvanich, S. Physicochemical changes of ‘Phulae” pineapple fruit treated with short-term anoxia during ambient storage. Food Chem. 2017, 228, 388–393. [Google Scholar] [CrossRef]
- Shehrawat, P.; Sindhu, N. Agricultural waste utilization for healthy environment and sustainable lifestyle. Agric. Biol. Res. 2015, 20, 110–114. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Use of agro-industrial wastes in solid-state fermentation processes. Ind. Waste 2012, 274, 121–139. [Google Scholar]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent developments on nanocellulose reinforced polymer nanocomposites: A review. Polymer 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Mutwil, M.; DeBolt, S.; Persson, S. Cellulose synthesis: A complex complex. Curr. Opin. Plant Biol. 2008, 11, 252–257. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothan, L.A.; Jacob, M.; Thomas, S.; Cvelbar, U.; Anandjiwala, R. Extraction of nanocellulose fibrils from lignocellulosic fibres: A novel approach. Carbohydr. Polym. 2011, 86, 1468–1475. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2009, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Saba, N.; Safwan, A.; Sanyang, M.L.; Mohammad, F.; Pervaiz, M.; Jawaid, M.; Alothman, O.Y.; Sain, M. Thermal and dynamic mechanical properties of cellulose nanofibers reinforced epoxy composites. Int. J. Biol. Macromol. 2017, 102, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.I.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Coelho, C.; Michelin, M.; Cerqueira, M.A.; Gonçalves, C.; Tonon, R.V.; Pastrana, L.M.; Freitas-Silva, O.; Vicente, A.A.; Cabral, L.M.; Teixeira, J.A. Cellulose nanocrystals from grape pomace: Production, properties and cytotoxicity assessment. Carbohydr. Polym. 2018, 192, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Reddy, J.P.; Rhim, J.-W. Extraction and characterization of cellulose microfibers from agricultural wastes of onion and garlic. J. Nat. Fibers 2018, 15, 465–473. [Google Scholar] [CrossRef]
- Wang, W.; Liu, C.; Huang, F.; Li, W.; Zheng, C. Preparation and characterization of nanocellulose from rapeseed hull. Oil Crop Sci. 2019, 4, 55–64. [Google Scholar] [CrossRef]
- Leite, A.L.M.P.; Zanon, C.D.; Menegalli, F.C. Isolation and characterization of cellulose nanofibers from cassava root bagasse and peelings. Carbohydr. Polym. 2017, 157, 962–970. [Google Scholar] [CrossRef]
- Rahman, N.H.A.; Chieng, B.W.; Ibrahim, N.A.; Rahman, N.A. Extraction and characterization of cellulose nanocrystals from tea leaf waste fibers. Polymer 2017, 9, 588. [Google Scholar] [CrossRef] [Green Version]
- Oun, A.; Rhim, J.-W. Isolation of cellulose nanocrystals from grain straws and their use for the preparation of carboxymethyl cellulose-based nanocomposite films. Carbohydr. Polym. 2016, 150, 187–200. [Google Scholar] [CrossRef]
- Sankhla, S.; Sardar, H.H.; Neogi, S. Greener extraction of highly crystalline and thermally stable cellulose micro-fibers from sugarcane bagasse for cellulose nano-fibrils preparation. Carbohydr. Polym. 2021, 251, 117030. [Google Scholar] [CrossRef]
- Technical Association of Pulp Paper Industry (TAPPI). TAPPI T222 Om-98: Acid-Insoluble Lignin in Wood and Pulp; TAPPI Press: Atlanta, GA, USA, 1999. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Mehanny, S.; Magd, E.E.A.-E.; Ibrahim, M.; Farag, M.; Gil-San-Millan, R.; Navarro, J.; Habbak, A.E.H.E.; El-Kashif, E. Extraction and characterization of nanocellulose from three types of palm residues. J. Mater. Res. Technol. 2021, 10, 526–537. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2011, 87, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Dutta, H. Characterization of nanocellulose extracted from short, medium and long grain rice husks. Ind. Crop. Prod. 2020, 154, 112627. [Google Scholar] [CrossRef]
- Dilamian, M.; Noroozi, B. A combined homogenization-high intensity ultrasonication process for individualizaion of cellulose micro-nano fibers from rice straw. Cellulose 2019, 26, 5831–5849. [Google Scholar] [CrossRef]
- Louis, A.C.F.; Venkatachalam, S. Energy efficient process for valorization of corn cob as a source for nanocrystalline cellulose and hemicellulose production. Int. J. Biol. Macromol. 2020, 163, 260–269. [Google Scholar] [CrossRef]
- Sasikala, M.; Umapathy, M.J. Preparation and characterization of pineapple leaf cellulose nanocrystal reinforced gelatin bio-nanocomposite with antibacterial banana leaf extract for application in food packaging. New J. Chem. 2018, 42, 19979–19986. [Google Scholar] [CrossRef]
- Leão, R.M.; Miléo, P.C.; Maia, J.M.; Luz, S. Environmental and technical feasibility of cellulose nanocrystal manufacturing from sugarcane bagasse. Carbohydr. Polym. 2017, 175, 518–529. [Google Scholar] [CrossRef]
- Reddy, J.P.; Rhim, J.-W. Isolation and characterization of cellulose nanocrystals from garlic skin. Mater. Lett. 2014, 129, 20–23. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Qi, X.; Chu, J.; Jia, L.; Kumar, A. Influence of different pretreatments on the structure and hydrolysis behavior of bamboo: A comparative study. Materials 2019, 12, 2570. [Google Scholar] [CrossRef] [Green Version]
- Beltramino, F.; Roncero, M.B.; Torres, A.L.; Vidal, T.; Valls, C. Optimization of sulfuric acid hydrolysis conditions for preparation of nanocrystalline cellulose from enzymatically pretreated fibers. Cellulose 2016, 23, 1777–1789. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Jiang, W.; Li, X.; Zhang, X.; Zhang, Y.; Li, M. Effect of steam pressure on chemical and structural properties of kenaf fibers during steam explosion process. Bioresources 2016, 11, 6590–6599. [Google Scholar] [CrossRef] [Green Version]
- Avolio, R.; Bonadies, I.; Capitani, D.; Errico, M.; Gentile, G.; Avella, M. A multitechnique approach to assess the effect of ball milling on cellulose. Carbohydr. Polym. 2012, 87, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Neto, W.P.F.; Silvério, H.A.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue—Soy hulls. Ind. Crop. Prod. 2012, 42, 480–488. [Google Scholar] [CrossRef]
- Mohaiyiddin, M.S.; Lin, O.H.; Owi, W.T.; Chan, C.H.; Chia, C.H.; Zakaria, S.; Villagracia, A.R.; Akil, H.M. Characterization of nanocellulose recovery from Elaeis guineensis frond for sustainable development. Clean Technol. Environ. Policy 2016, 18, 2503–2512. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Perumal, A.B.; Sellamuthu, P.S.; Nambiar, R.B.; Sadiku, E.R.; Phiri, G.; Jayaramudu, J. Effects of multiscale rice straw (Oryza sativa) as reinforcing filler in montmorillonite-polyvinyl alcohol biocomposite packaging film for enhancing the storability of postharvest mango fruit (Mangifera indica L.). Appl. Clay Sci. 2018, 158, 1–10. [Google Scholar] [CrossRef]
- Chi, X.; Liu, C.; Bi, Y.-H.; Yu, G.; Zhang, Y.; Wang, Z.; Li, B.; Cui, Q. A clean and effective potassium hydroxide pretreatment of corncob residue for the enhancement of enzymatic hydrolysis at high solids loading. RSC Adv. 2019, 9, 11558–11566. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Zhao, S.; Yang, S.; Ding, S.-Y. Lignin plays a negative role in the biochemical process for producing lignocellulosic biofuels. Curr. Opin. Biotechnol. 2014, 27, 38–45. [Google Scholar] [CrossRef]
- Merci, A.; Urbano, A.; Grossmann, M.V.E.; Tischer, C.; Mali, S. Properties of microcrystalline cellulose extracted from soybean hulls by reactive extrusion. Food Res. Int. 2015, 73, 38–43. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, Y.; Guo, Y.; Yue, J. Isolation and Characterization of nanocellulose with a novel shape from walnut (Juglans regia L.) shell agricultural waste. Polymers 2019, 11, 1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussin, M.H.; Pohan, N.A.; Garba, Z.N.; Kassim, M.J.; Rahim, A.A.; Brosse, N.; Yemloul, M.; Fazita, M.N.; Haafiz, M.M. Physicochemical of microcrystalline cellulose from oil palm fronds as potential methylene blue adsorbents. Int. J. Biol. Macromol. 2016, 92, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Razali, N.; Salit, M.S.; Jawaid, M.; Ishak, M.R.; Lazim, Y. A Study on chemical composition, physical, tensile, morphological, and thermal properties of roselle fibre: Effect of fibre maturity. Bioresources 2014, 10, 1803–1824. [Google Scholar] [CrossRef] [Green Version]




| Samples | Yield (% w/w) | Ash (% w/w) | Lignin (% w/w) | Hemicellulose (% w/w) | α-Cellulose (% w/w) |
|---|---|---|---|---|---|
| RS | - | 9.96 ± 0.14 a | 21.63 ± 0.71 a | 31.01 ± 0.99 b | 45.45 ± 1.35 c |
| CC | - | 3.17 ± 0.13 d | 14.93 ± 0.36 c | 27.78 ± 1.65 c | 45.81 ± 0.61 c |
| PL | - | 6.35 ± 0.20 b | 27.25 ± 0.43 b | 31.05 ± 1.46 b | 35.35 ± 1.01 d |
| PP | - | 4.79 ± 0.21 c | 12.70 ± 1.45 d | 44.15 ± 2.63 a | 33.18 ± 1.47 e |
| AE-RSC | 32.26 ± 1.34 b | 0.68 ± 0.10 e | 0.89 ± 0.03 e | 13.51 ± 0.45 d | 79.19 ± 0.69 b |
| AE-CCC | 38.18 ± 0.66 a | 0.42 ± 0.03 f | 0.58 ± 0.06 e | 9.61 ± 0.75 e | 82.69 ± 1.10 a |
| AE-PLC | 16.60 ± 1.42 c | 0.87 ± 0.03 e | 0.68 ± 0.10 e | 13.24 ± 0.21 d | 78.64 ± 0.47 b |
| AE-PPC | 9.05 ± 0.07 d | 0.77 ± 0.03 e | 0.27 ± 0.04 e | 14.60 ± 1.85 d | 78.07 ± 1.44 b |
| Samples | CI (%) | Tonset (°C) | Tmax (°C) | Residue at 600 °C (%) |
|---|---|---|---|---|
| RS | 33.57 | - | - | - |
| CC | 21.40 | - | - | - |
| PL | 20.73 | - | - | - |
| PP | 2.38 | - | - | - |
| AE-RSC | 66.10 | 330.53 | 360.00 | 15.24 |
| AE-CCC | 69.45 | 334.64 | 364.67 | 13.37 |
| AE-PLC | 51.75 | 331.37 | 358.00 | 15.44 |
| AE-PPC | 44.58 | 323.07 | 357.67 | 18.40 |
| COM-C | 80.14 | 321.10 | 357.00 | 12.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romruen, O.; Karbowiak, T.; Tongdeesoontorn, W.; Shiekh, K.A.; Rawdkuen, S. Extraction and Characterization of Cellulose from Agricultural By-Products of Chiang Rai Province, Thailand. Polymers 2022, 14, 1830. https://doi.org/10.3390/polym14091830
Romruen O, Karbowiak T, Tongdeesoontorn W, Shiekh KA, Rawdkuen S. Extraction and Characterization of Cellulose from Agricultural By-Products of Chiang Rai Province, Thailand. Polymers. 2022; 14(9):1830. https://doi.org/10.3390/polym14091830
Chicago/Turabian StyleRomruen, Orapan, Thomas Karbowiak, Wirongrong Tongdeesoontorn, Khursheed Ahmad Shiekh, and Saroat Rawdkuen. 2022. "Extraction and Characterization of Cellulose from Agricultural By-Products of Chiang Rai Province, Thailand" Polymers 14, no. 9: 1830. https://doi.org/10.3390/polym14091830