Long-Term Performance and Stability of Interlayer-Free Mesoporous Silica Membranes for Wetland Saline Water Pervaporation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Thin Film Fabrication
2.2. Membrane Characterization
2.3. Desalination Performance and Long-Term Stability
3. Results and Discussion
3.1. Xerogel Surface Chemistry
| Sample Code | BET Surface Area (m2·g−1) | Pore Volume (10−6 m3·g−1) | Average Pore Diameter (nm) | Thickness (nm) | Ref. |
|---|---|---|---|---|---|
| Pure silica (RTP) | 272 | 0.17 | 2.50 | ~1000 | This work |
| Pure silica (CTP) | 402 | 0.221 | 2.70 | 400 | [27] |
3.2. Membrane Morphology
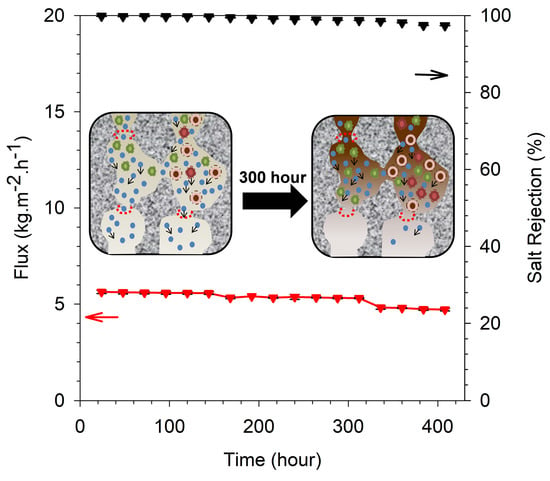
3.3. Desalination Performance and Long-Term Stability
3.4. Long-Term Stability Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Osaki, M.; Setiadi, B.; Takahashi, H.; Evri, M. Tropical Peatland Ecosystems; Springer: Tokyo, Japan, 2016. [Google Scholar]
- Rahma, A.; Elma, M.; Rampun, E.L.A.; Pratiwi, A.E.; Rakhman, A.; Fitriani, F. Rapid Thermal Processing and Long Term Stability of Interlayer-free Silica-P123 Membranes for Wetland Saline Water Desalination. J. Adv. Res. Fluid Mech. Therm. Sci. 2020, 71, 1–9. [Google Scholar] [CrossRef]
- Zein, R.; Mukhlis; Swesti, N.; Novita, L.; Novrian, E.; Ningsih, S.; Syukri. Peat water treatment by using multi soil layering (msl) method. Der Pharma Chem. 2016, 8, 254–261. [Google Scholar]
- Wang, D.K.; Elma, M.; Motuzas, J.; Hou, W.-C.; Schmeda-Lopez, D.R.; Zhang, T.; Zhang, X. Physicochemical and photocatalytic properties of carbonaceous char and titania composite hollow fibers for wastewater treatment. Carbon 2016, 109, 182–191. [Google Scholar] [CrossRef]
- Duke, M.C.; Mee, S.; Da Costa, J.C.D. Performance of porous inorganic membranes in non-osmotic desalination. Water Res. 2007, 41, 3998–4004. [Google Scholar] [CrossRef] [PubMed]
- Elma, M.; Pratiwi, A.E.; Rahma, A.; Rampun, E.L.A.; Handayani, N. The Performance of Membranes Interlayer-Free Silica-Pectin Templated for Seawater Desalination via Pervaporation Operated at High Temperature of Feed Solution. Mater. Sci. Forum 2020, 981, 349–355. [Google Scholar] [CrossRef]
- Sumardi, A.; Elma, M.; Rampun, E.L.A.; Lestari, A.E.; Assyaifi, Z.L.; Darmawan, A.; Yanto, D.H.Y.; Syauqiah, I.; Mawaddah, Y.; Wati, L.S. Designing a mesoporous hybrid organo-silica thin film prepared from an organic catalyst. Membr. Technol. 2021, 2021, 5–8. [Google Scholar] [CrossRef]
- Da Costa, J.C.D.; Lu, G.; Rudolph, V.; Lin, Y. Novel molecular sieve silica (MSS) membranes: Characterisation and permeation of single-step and two-step sol–gel membranes. J. Membr. Sci. 2002, 198, 9–21. [Google Scholar] [CrossRef]
- Wijaya, S.; Duke, M.C.; Da Costa, J.C.D. Carbonised template silica membranes for desalination. Desalination 2009, 236, 291–298. [Google Scholar] [CrossRef]
- Rahma, A.; Elma, M.; Pratiwi, A.E.; Rampun, E.L. Performance of interlayer-free pectin template silica membranes for brackish water desalination. Membr. Technol. 2020, 2020, 7–11. [Google Scholar] [CrossRef]
- Elma, M.; Saputro, G.S. Performance of Cobalt-Silica Membranes through Pervaporation Process with Different Feed Solution Concentrations. Mater. Sci. Forum 2020, 981, 342–348. [Google Scholar] [CrossRef]
- De Vos, R.M.; Maier, W.F.; Verweij, H. Hydrophobic silica membranes for gas separation. J. Membr. Sci. 1999, 158, 277–288. [Google Scholar] [CrossRef]
- Wang, D.K.; Da Costa, J.C.D.; Smart, S. Development of rapid thermal processing of tubular cobalt oxide silica membranes for gas separations. J. Membr. Sci. 2014, 456, 192–201. [Google Scholar] [CrossRef]
- Wang, S.; Wang, D.; Smart, S.; da Costa, J.C.D. Improved stability of ethyl silicate interlayer-free membranes by the rapid thermal processing (RTP) for desalination. Desalination 2017, 402, 25–32. [Google Scholar] [CrossRef]
- Rampun, E.L.; Elma, M.; Rahma, A.; Pratiwi, A.E. Interlayer-free silica–pectin membrane for sea-water desalination. Membr. Technol. 2019, 2019, 5–9. [Google Scholar] [CrossRef]
- Lin, C.X.C.; Ding, L.P.; Smart, S.; da Costa, J.C.D. Cobalt oxide silica membranes for desalination. J. Colloid Interface Sci. 2012, 368, 70–76. [Google Scholar] [CrossRef]
- Ladewig, B.P.; Tan, Y.H.; Lin, C.X.C.; Ladewig, K.; Diniz da Costa, J.C.; Smart, S. Preparation, Characterization and Performance of Templated Silica Membranes in Non-Osmotic Desalination. Materials 2011, 4, 845–856. [Google Scholar] [CrossRef]
- Elma, M.; Riskawati, N. Silica Membranes for Wetland Saline Water Desalination: Performance and Long Term Stability. IOP Conf. Ser. Earth Environ. Sci. 2018, 175, 012006. [Google Scholar] [CrossRef]
- Bertoluzza, A.; Fagnano, C.; Morelli, M.A.; Gottardi, V.; Guglielmi, M. Raman and infrared spectra on silica gel evolving toward glass. J. Non-Cryst. Solids 1982, 48, 117–128. [Google Scholar] [CrossRef]
- Brinker, C.; Keefer, K.; Schaefer, D.; Ashley, C. Sol-gel transition in simple silicates. J. Non-Cryst. Solids 1982, 48, 47–64. [Google Scholar] [CrossRef]
- Brinker, C.; Keefer, K.; Schaefer, D.; Assink, R.; Kay, B.; Ashley, C. Sol-gel transition in simple silicates II. J. Non-Cryst. Solids 1984, 63, 45–59. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol. Gel Science: The Physics and Chemistry of Sol-Gel Processing, 1st ed; Academic Press, Inc.: San Diego, CA, USA, 1990. [Google Scholar]
- Pratiwi, A.E.; Elma, M.; Rahma, A.; Rampun, E.L.A.; Saputro, G.S. Deconvolution of pectin carbonised template silica thin-film: Synthesis and characterisation. Membr. Technol. 2019, 2019, 5–8. [Google Scholar] [CrossRef]
- Elma, M.; Mujiyanti, D.R.; Ismail, N.M.; Bilad, M.R.; Rahma, A.; Rahman, S.K.; Fitriani, F.; Rakhman, A.; Rampun, E.L.A. Development of Hybrid and Templated Silica-P123 Membranes for Brackish Water Desalination. Polymers 2020, 12, 2644. [Google Scholar] [CrossRef]
- Elma, M.; Yacou, C.; Da Costa, J.C.D.; Wang, D.K. Performance and Long Term Stability of Mesoporous Silica Membranes for Desalination. Membranes 2013, 3, 136–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Wang, D.K.; Motuzas, J.; Smart, S.; da Costa, J.C.D. Rapid thermal treatment of interlayer-free ethyl silicate 40 derived membranes for desalination. J. Membr. Sci. 2016, 516, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Elma, M.; Rahma, A.; Pratiwi, A.E.; Rampun, E.L. Coagulation as pretreatment for membrane-based wetland saline water desalination. Asia-Pac. J. Chem. Eng. 2020, 15, e2461. [Google Scholar] [CrossRef]
- Pradhana, E.A.; Elma, M.; Othman, M.H.D.; Huda, N.; Ul-Haq, M.D.; Rampun, E.L.; Rahma, A. The Functionalization Study of PVDF/TiO2 Hollow Fibre Membranes Under Vacuum Calcination Exposure. J. Phys. Conf. Ser. 2021, 1912, 012035. [Google Scholar] [CrossRef]
- Elma, M.; Rampun, E.L.; Rahma, A.; Assyaifi, Z.L.; Sumardi, A.; Lestari, A.E.; Saputro, G.S.; Bilad, M.R.; Darmawan, A. Carbon templated strategies of mesoporous silica applied for water desalination: A review. J. Water Process Eng. 2020, 38, 101520. [Google Scholar] [CrossRef]
- Mustalifah, F.R.; Rahma, A.; Mahmud; Sunardi; Elma, M. Chemical cleaning to evaluate the performance of silica-pectin membrane on acid mine drainage desalination. IOP Conf. Series Mater. Sci. Eng. 2021, 1195, 012057. [Google Scholar] [CrossRef]
- Gao, X.; Bonilla, M.; da Costa, J.C.D.; Bhatia, S. The transport of gases in a mesoporous γ-alumina supported membrane. J. Membr. Sci. 2013, 428, 357–370. [Google Scholar] [CrossRef]
- Gao, X.; da Costa, J.C.D.; Bhatia, S.K. The transport of gases in a supported mesoporous silica membrane. J. Membr. Sci. 2013, 438, 90–104. [Google Scholar] [CrossRef]
- Cheraitia, A.; Ayral, A.; Julbe, A.; Rouessac, V.; Satha, H. Synthesis and characterization of microporous silica–alumina membranes. J. Porous Mater. 2009, 17, 259–263. [Google Scholar] [CrossRef]
- Uhlmann, D.; Smart, S.; Da Costa, J.C.D. High temperature steam investigation of cobalt oxide silica membranes for gas separation. Sep. Purif. Technol. 2010, 76, 171–178. [Google Scholar] [CrossRef]
- Xu, R.; Wang, J.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Development of Robust Organosilica Membranes for Reverse Osmosis. Langmuir 2011, 27, 13996–13999. [Google Scholar] [CrossRef]
- Elma, M.; Assyaifi, Z.L. Desalination Process via Pervaporation of Wetland Saline Water. IIOP Conf. Ser. Earth Environ. Sci. 2018, 175, 012009. [Google Scholar] [CrossRef]
- Li, L.; Dong, J.; Nenoff, T.M.; Lee, R. Desalination by reverse osmosis using MFI zeolite membranes. J. Membr. Sci. 2004, 243, 401–404. [Google Scholar] [CrossRef]
- Burneau, A.E.; Gallas, J.-P.; Legrand, A.E. (Eds.) The Surface Properties of Silicas; Wiley: Chichester, UK, 1998. [Google Scholar]
- Haan, H.D.; Boe, T.D. Applicability of Light Absorbance and Fluorescence as Measures Of Concentration and Molecular Size of Dissolved Organic Carbon in Humic Lake Tjeukemeer. Water Resources 1987, 21, 731–734. [Google Scholar]
- Yunos, M.Z.; Harun, Z.; Basri, H.; Ismail, A.F. Studies on fouling by natural organic matter (NOM) on polysulfone membranes: Effect of polyethylene glycol (PEG). Desalination 2014, 333, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Jeong, K.; Kim, D.G.; Ko, S.O. Adsorption characteristics of Effluent Organic Matter and Natural Organic Matter by Carbon Based Nanomaterials. KSCE J. Civ. Eng. 2016, 21, 119–126. [Google Scholar] [CrossRef]
- Aryanti, P.; Joscarita, S.R.; Wardani, A.K.; Subagjo, S.; Ariono, D.; Wenten, I.G. The Influence of PEG400 and Acetone on Polysulfone Membrane Morphology and Fouling Behaviour. J. Eng. Technol. Sci. 2016, 48, 135–149. [Google Scholar] [CrossRef] [Green Version]
- Ariono, D.; Aryanti, P.T.P.; Subagjo, S.; Wenten, I.G. The Effect of Polymer Concentration on Flux Stability of Polysulfone Membrane. In Proceedings of the International Conference on Engineering Science and Nanotechnology (ICESNANO 2016), Solo, Indonesia, 3–5 August 2016; American Institute of Physics: Melville, NY, USA, 2017; pp. 1–10. [Google Scholar]
- Lee, J.; Vigneswaran, S.; Zhang, Y.; Reddy, R.S.P.R.; Liu, Z. Effective natural organic matter removal in pond water by carbon nanotube membrane with flocculation/adsorption. Water Supply 2017, 17, 1080–1087. [Google Scholar] [CrossRef] [Green Version]
- Waqas, S.; Bilad, M.R.; Man, Z.B.; Suleman, H.; Nordin, N.A.H.; Jaafar, J.; Othman, M.H.D.; Elma, M. An energy-efficient membrane rotating biological contactor for wastewater treatment. J. Clean. Prod. 2020, 282, 124544. [Google Scholar] [CrossRef]
- Razak, N.N.A.N.; Rahmawati, R.; Bilad, M.R.; Pratiwi, A.E.; Elma, M.; Nawi, N.I.M.; Jaafar, J.; Lam, M.K. Finned spacer for enhancing the impact of air bubbles for membrane fouling control in Chlorella vulgaris filtration. Bioresour. Technol. Rep. 2020, 11, 100429. [Google Scholar] [CrossRef]
- Aryanti, P.T.P.; Khoiruddin, I.; Wenten, I.G. Influence of Additives on Polysulfone-Based Ultrafiltration Membrane Performance during Peat Water Filtration. J. Water Sustain. 2013, 3, 85–96. [Google Scholar] [CrossRef]
- Schillo, M.; Park, I.-S.; Chiu, W.; Verweij, H. Rapid thermal processing of inorganic membranes. J. Membr. Sci. 2010, 362, 127–133. [Google Scholar] [CrossRef]
- Wang, D.K.; Motuzas, J.; Da Costa, J.C.D.; Smart, S. Rapid thermal processing of tubular cobalt oxide silica membranes. Int. J. Hydrog. Energy 2013, 38, 7394–7399. [Google Scholar] [CrossRef]
- Song, Y.; Dong, B.; Gao, N.; Ma, X. Powder Activated Carbon Pretreatment of a Microfiltration Membrane for the Treatment of Surface Water. Int. J. Environ. Res. Public Heal. 2015, 12, 11269–11277. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Guo, L. Molecular size-dependent abundance and composition of dissolved organic matter in river, lake and sea waters. Water Res. 2017, 117, 115–126. [Google Scholar] [CrossRef]
- Yang, H.; Elma, M.; Wang, D.; Motuzas, J.; da Costa, J.C.D. Interlayer-free hybrid carbon-silica membranes for processing brackish to brine salt solutions by pervaporation. J. Membr. Sci. 2017, 523, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Aryanti, P.T.P.; Subagjo, S.; Ariono, D.; Wenten, I.G. Fouling and Rejection Characteristic of Humic Substances in Polysulfone Ultrafiltration Membrane. J. Membr. Sci. Res. 2015, 1, 41–45. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elma, M.; Bilad, M.R.; Pratiwi, A.E.; Rahma, A.; Asyyaifi, Z.L.; Hairullah, H.; Syauqiah, I.; Arifin, Y.F.; Lestari, R.A. Long-Term Performance and Stability of Interlayer-Free Mesoporous Silica Membranes for Wetland Saline Water Pervaporation. Polymers 2022, 14, 895. https://doi.org/10.3390/polym14050895
Elma M, Bilad MR, Pratiwi AE, Rahma A, Asyyaifi ZL, Hairullah H, Syauqiah I, Arifin YF, Lestari RA. Long-Term Performance and Stability of Interlayer-Free Mesoporous Silica Membranes for Wetland Saline Water Pervaporation. Polymers. 2022; 14(5):895. https://doi.org/10.3390/polym14050895
Chicago/Turabian StyleElma, Muthia, Muhammad Roil Bilad, Amalia Enggar Pratiwi, Aulia Rahma, Zaini Lambri Asyyaifi, Hairullah Hairullah, Isna Syauqiah, Yulian Firmana Arifin, and Riani Ayu Lestari. 2022. "Long-Term Performance and Stability of Interlayer-Free Mesoporous Silica Membranes for Wetland Saline Water Pervaporation" Polymers 14, no. 5: 895. https://doi.org/10.3390/polym14050895