Red Seaweed (Gracilaria verrucosa Greville) Based Polyurethane as Adsorptive Membrane for Ammonia Removal in Water
Abstract
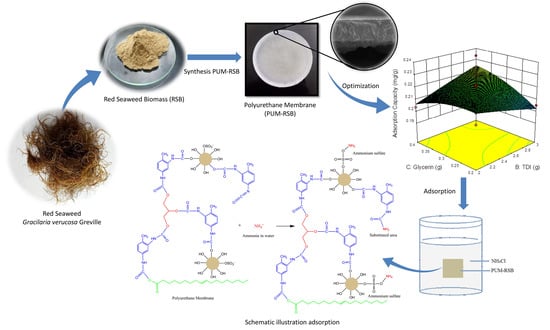
:1. Introduction
2. Materials and Methods
2.1. Instruments and Materials
2.2. Treatment Design
2.3. Polyurethane Membrane Fabrication
2.4. Adsorption Process
2.5. Adsorption Capacity and Efficiency
2.6. Adsorption Isotherm
2.7. Adsorption Kinetics
3. Results and Discussion
3.1. Ammonia Adsorption
3.2. Statistical Design Model
3.3. Polyurethane Membrane Optimization
3.4. Ammonia Adsorption Using Optimal Polyurethane Membrane
3.5. Adsorption Isotherm
3.6. Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.D.; Macosko, C.W.; Davis, H.T. Effect of Silicone Surfactant on Air Flow of Flexible Polyurethane Foams. In ACS Symposium Series; American Chemical Society (ACS): Washington, DC, USA, 1997; pp. 130–142. [Google Scholar]
- Howard, G.T. Biodegradation of polyurethane: A review. Int. Biodeterior. Biodegrad. 2002, 49, 245–252. [Google Scholar] [CrossRef]
- Almeida, J.H.S., Jr.; Bertuol, D.A.; Meneguzzi, A.; Ferreira, C.A.; Amado, F.D.R. Castor oil and commercial thermoplastic polyurethane membranes modified with polyaniline: A comparative study. Mater. Res. 2013, 16, 860–866. [Google Scholar] [CrossRef] [Green Version]
- Marlina, S.; Nurman, S. Synthesis and Characterization New Polyurethane Membrane From Hydroxylated Rubber Seed Oil. Orient. J. Chem. 2017, 33, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; MTR: Newark, CA, USA, 2012. [Google Scholar]
- Nunes, S.P.; Peinemann, K.-V. Membrane Technology: In the Chemical Industry; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2006. [Google Scholar]
- Marlina, M. Pemanfaatan asam lemak bebas teroksidasi dari minyak jarak untuk sintesis membran poliuretan. J. Rekayasa Kim. Lingkung. 2007, 6, 67–70. [Google Scholar]
- Marlina, M. Sintesis Membran Poliuretan dari Karagenan dan 2, 4 Toylulene Diisosianat. J. Rekayasa Kim. Lingkung. 2010, 7, 138–148. [Google Scholar]
- Huang, S.-L.; Lai, J.-Y. On the gas permeability of hydroxyl terminated polybutadiene based polyurethane membranes. J. Membr. Sci. 1995, 105, 137–145. [Google Scholar] [CrossRef]
- Iqhrammullah, M.; Mustafa, I. The application of chitosan modified polyurethane foam adsorbent. Rasayan J. Chem. 2019, 12, 494–501. [Google Scholar]
- Adam, M.R.; Matsuura, T.; Othman, M.H.D.; Puteh, M.H.; Pauzan, M.A.B.; Ismail, A.; Mustafa, A.; Rahman, M.A.; Jaafar, J.; Abdullah, M.S. Feasibility study of the hybrid adsorptive hollow fibre ceramic membrane (HFCM) derived from natural zeolite for the removal of ammonia in wastewater. Process. Saf. Environ. Prot. 2019, 122, 378–385. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Puteh, M.H.; Ismail, A.; Mustafa, A.; Rahman, M.A.; Jaafar, J. Impact of sintering temperature and pH of feed solution on adsorptive removal of ammonia from wastewater using clinoptilolite based hollow fibre ceramic membrane. J. Water Process. Eng. 2020, 33, 101063. [Google Scholar] [CrossRef]
- Iqhrammullah, M.; Saleha, S.; Maulina, F.P.; Idroes, R. Polyurethane film prepared from ball-milled algal polyol particle and activated carbon filler for NH3–N removal. Heliyon 2020, 6, e04590. [Google Scholar] [CrossRef]
- Ramírez, M.; Gómez, J.M.; Aroca, G.; Cantero, D. Removal of ammonia by immobilized Nitrosomonas europaea in a biotrickling filter packed with polyurethane foam. Chemosphere 2009, 74, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-J.; Liu, Y.-W.; Cheng, H.-H.; Ke, C.-W.; Lin, T.-F.; Whang, L.-M. Biological pre-treatment system for ammonia removal from slightly contaminated river used as a drinking water source. Process. Saf. Environ. Prot. 2020, 147, 385–391. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Abu Samah, R.; Puteh, M.H.; Ismail, A.; Mustafa, A.; Rahman, M.A.; Jaafar, J. Current trends and future prospects of ammonia removal in wastewater: A comprehensive review on adsorptive membrane development. Sep. Purif. Technol. 2019, 213, 114–132. [Google Scholar] [CrossRef]
- Song, W.; Li, Z.; Ding, Y.; Liu, F.; You, H.; Qi, P.; Wang, F.; Li, Y.; Jin, C. Performance of a novel hybrid membrane bioreactor for treating saline wastewater from mariculture: Assessment of pollutants removal and membrane filtration performance. Chem. Eng. J. 2018, 331, 695–703. [Google Scholar] [CrossRef]
- Moreno, J.; Arregui, F.J.; Matias, I.R. Fiber optic ammonia sensing employing novel thermoplastic polyurethane membranes. Sens. Actuators B Chem. 2005, 105, 419–424. [Google Scholar] [CrossRef]
- Nurman, S.; Saiful, S.; Ginting, B.; Rahmi, R.; Marlina, M.; Wibisono, Y. Synthesis of Polyurethane Membranes Derived from Red Seaweed Biomass for Ammonia Filtration. Membrancs 2021, 11, 668. [Google Scholar] [CrossRef]
- Nurman, S.; Saiful, S.; Ginting, B.; Rahmi, R.; Marlina, M. Optimization of Polyurethane Membrane Physical Characteristics of Red Seaweed Biomass Using a Box-Behnken Design. Indones. J. Chem. 2021, 21, 932–941. [Google Scholar] [CrossRef]
- Saiful, M.; Saleha, S.; Rahmi, F. Sintesis Membran Poliuretan Berbasis Bahan Alam; Syiah Kuala University Press: Banda Aceh, Indonesia, 2017. [Google Scholar]
- Kasanah, N.; Setyadi; Triyanto; Trialfhianty, T.I. Rumput Laut Indonesia; Gadjah Mada University Press: Yogyakarta, Indonesia, 2018. [Google Scholar]
- Sedayu, B.B.; Cran, M.J.; Bigger, S. A Review of Property Enhancement Techniques for Carrageenan-based Films and Coatings. Carbohydr. Polym. 2019, 216, 287–302. [Google Scholar] [CrossRef]
- Agrahari, G.K.; Shukla, S.K.; Verma, N.; Bhattacharya, P.K. Model prediction and experimental studies on the removal of dissolved NH3 from water applying hollow fiber membrane contactor. J. Membr. Sci. 2012, 390, 164–174. [Google Scholar] [CrossRef]
- Cheng, H.; Zhu, Q.; Xing, Z. Adsorption of ammonia nitrogen in low temperature domestic wastewater by modification bentonite. J. Clean. Prod. 2019, 233, 720–730. [Google Scholar] [CrossRef]
- Li, R.; Shan, Z. Study on structure-induced heat transfer capabilities of waterborne polyurethane membranes. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 598, 124879. [Google Scholar] [CrossRef]
- Melnig, V.; Apostu, M.; Tura, V.; Ciobanu, C. Optimization of polyurethane membranes: Morphology and structure studies. J. Membr. Sci. 2005, 267, 58–67. [Google Scholar] [CrossRef]
- Tian, H.; Li, Z.; Lu, P.; Wang, Y.; Jia, C.; Wang, H.; Liu, Z.; Zhang, M. Starch and castor oil mutually modified, cross-linked polyurethane for improving the controlled release of urea. Carbohydr. Polym. 2021, 251, 117060. [Google Scholar] [CrossRef] [PubMed]
- SNI.06-6989.30-2005; Air Dan Air Limbah—Bagian 30: Cara Uji Kadar Amonia Dengan Spektrofotometer Secara Fenat. Badan Standardisasi Nasional: Jakarta, Indonesia, 2005.
- Wongcharee, S.; Aravinthan, V.; Erdei, L. Removal of natural organic matter and ammonia from dam water by enhanced coagulation combined with adsorption on powdered composite nano-adsorbent. Environ. Technol. Innov. 2020, 17, 100557. [Google Scholar] [CrossRef]
- Zhu, W.; Lin, Y.; Kang, W.; Quan, H.; Zhang, Y.; Chang, M.; Wang, K.; Zhang, M.; Zhang, W.; Li, Z.; et al. An aerogel adsorbent with bio-inspired interfacial adhesion between graphene and MoS2 sheets for water treatment. Appl. Surf. Sci. 2020, 512, 145717. [Google Scholar] [CrossRef]
- Oh, H.-J.; Jang, H.R.; Jung, K.Y.; Kim, J.-H. Evaluation of surface area of mesoporous silica adsorbents for separation and purification of paclitaxel. Microporous Mesoporous Mater. 2013, 180, 109–113. [Google Scholar] [CrossRef]
- Tekindal, M.A.; Bayrak, H.; Ozkaya, B.; Genc, Y. Box-Behnken Experimental Design in Factorial Experiments: The Importance of Bread For Nutrition and Health. Turk. J. Field Crops 2012, 17, 115–123. [Google Scholar]
- Yulia, R.; Meilina, H.; Adisalamun, A.; Darmadi, D. Aplikasi Metode Advance Oxidation Process (AOP) Fenton pada Pengolahan Limbah Cair Pabrik Kelapa Sawit Application of Advanced Oxidation Process (AOP) Fenton on Palm Oil Mill Effluent Treatment. J. Rekayasa Kim. Lingkung. 2016, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chelladurai, S.J.S.; Murugan, K.; Ray, A.P.; Upadhyaya, M.; Narasimharaj, V.; Gnanasekaran, S. Optimization of process parameters using response surface methodology: A review. Mater. Today Proc. 2021, 37, 1301–1304. [Google Scholar] [CrossRef]
- Zhao, Z.; Bermudez, S.C.; Ilyas, A.; Muylaert, K.; Vankelecom, I.F. Optimization of negatively charged polysulfone membranes for concentration and purification of extracellular polysaccharides from Arthrospira platensis using the response surface methodology. Sep. Purif. Technol. 2020, 252, 117385. [Google Scholar] [CrossRef]
- Mahdavi, H.; Razmi, F.; Shahalizade, T. Polyurethane TFC nanofiltration membranes based on interfacial polymerization of poly(bis-MPA) and MDI on the polyethersulfone support. Sep. Purif. Technol. 2016, 162, 37–44. [Google Scholar] [CrossRef]
- Chaabane, L.; Beyou, E.; El Ghali, A.; Baouab, M.H.V. Comparative studies on the adsorption of metal ions from aqueous solutions using various functionalized graphene oxide sheets as supported adsorbents. J. Hazard. Mater. 2020, 389, 121839. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; Sui, H.; He, L.; Yuan, W.; Han, Z. A Novel Polymeric Adsorbent Embedded with Phase Change Materials (PCMs) Microcapsules: Synthesis and Application. Nanomaterials 2019, 9, 736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limousin, G.; Gaudet, J.-P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Padmesh, T.; Palanivelu, K.; Velan, M. Biosorption of nickel (II) ions onto Sargassum wightii: Application of two-parameter and three-parameter isotherm models. J. Hazard. Mater. 2006, 133, 304–308. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Emik, S.; Öngen, A.; Özcan, K.H.; Aydın, S. Modelling of adsorption kinetic processes—Errors, theory and application. In Advanced Sorption Process Applications; IntechOpen: London, UK, 2019; pp. 187–206. [Google Scholar]
- Caballé-Serrano, J.; Abdeslam-Mohamed, Y.; Munar-Frau, A.; Fujioka-Kobayashi, M.; Hernández-Alfaro, F.; Miron, R. Adsorption and release kinetics of growth factors on barrier membranes for guided tissue/bone regeneration: A systematic review. Arch. Oral Biol. 2019, 100, 57–68. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Shirazian, S.; Ashrafizadeh, S.N. Simulation of ammonia removal from industrial wastewater streams by means of a hollow-fiber membrane contactor. Desalination 2012, 285, 383–392. [Google Scholar] [CrossRef] [Green Version]










| Factor | Parameter | Levels | ||
|---|---|---|---|---|
| Low (–) | Medium (0) | Height (+) | ||
| x1 | RSB (g) | 0.1 | 0.2 | 0.3 |
| x2 | TDI (g) | 2.0 | 2.5 | 3.0 |
| x3 | Glycerin (g) | 0.2 | 0.3 | 0.4 |
| Run | Factor 1 A: RSB (g) | Factor 2 B: TDI (g) | Factor 3 C: Glycerin (g) | Response Adsorption Capacity (mg/g) | Response Adsorption Efficiency (%) |
|---|---|---|---|---|---|
| 1 | 0.2 | 3.0 | 0.4 | 0.233 | 16.2 |
| 2 | 0.2 | 2.5 | 0.3 | 0.222 | 11.8 |
| 3 | 0.3 | 2.5 | 0.4 | 0.208 | 6.0 |
| 4 | 0.2 | 2.0 | 0.2 | 0.208 | 5.9 |
| 5 | 0.2 | 3.0 | 0.2 | 0.205 | 4.6 |
| 6 | 0.1 | 2.5 | 0.4 | 0.214 | 8.5 |
| 7 | 0.2 | 2.5 | 0.3 | 0.212 | 7.6 |
| 8 | 0.1 | 2.0 | 0.3 | 0.228 | 14.2 |
| 9 | 0.3 | 2.0 | 0.3 | 0.210 | 6.9 |
| 10 | 0.1 | 2.5 | 0.2 | 0.213 | 8.3 |
| 11 | 0.3 | 2.5 | 0.2 | 0.209 | 6.6 |
| 12 | 0.2 | 2.5 | 0.3 | 0.199 | 1.5 |
| 13 | 0.2 | 2.0 | 0.4 | 0.204 | 3.9 |
| 14 | 0.3 | 3.0 | 0.3 | 0.209 | 6.5 |
| 15 | 0.2 | 2.5 | 0.3 | 0.220 | 11.2 |
| 16 | 0.2 | 2.5 | 0.3 | 0.231 | 15.1 |
| 17 | 0.1 | 3.0 | 0.3 | 0.211 | 7.1 |
| Source | 2FI | Linear | Quadratic | Cubic |
|---|---|---|---|---|
| Std. Dev | 0.010 | 0.010 | 0.012 | 0.012 |
| R-Square | 0.340 | 0.120 | 0.389 | 0.618 |
| Adj R-Square | −0.056 | −0.084 | −0.396 | −0.527 |
| Pred R-Square | −0.760 | −0.451 | −3.257 | N/A |
| Adeq Precision | 3.444 | 2.653 | 2.506 | 2.838 |
| PRESS | 2.58 × 10−3 | 2.21 × 10−3 | 6.49 × 10−3 | N/A |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | Characterization |
|---|---|---|---|---|---|---|
| Model | 5.937 × 10−4 | 9 | 6.597 × 10−5 | 0.50 | 0.838 | Not significant |
| A-RSB | 1.054 × 10−4 | 1 | 1.054 × 10−4 | 0.79 | 0.403 | |
| B-TDI | 9.344 × 10−6 | 1 | 9.344 × 10−6 | 0.07 | 0.799 | |
| C-Glycerin | 6.764 × 10−5 | 1 | 6.764 × 10−5 | 0.51 | 0.499 | |
| AB | 6.841 × 10−5 | 1 | 6.841 × 10−5 | 0.51 | 0.496 | |
| AC | 8.625 × 10−7 | 1 | 8.625 × 10−7 | 6.486 × 10−3 | 0.938 | |
| BC | 2.671 × 10−4 | 1 | 2.671 × 10−4 | 2.01 | 0.199 | |
| A2 | 1.213 × 10−5 | 1 | 1.213 × 10−5 | 0.09 | 0.771 | |
| B2 | 7.310 × 10−7 | 1 | 7.310 × 10−7 | 5.497 × 10−3 | 0.943 | |
| C2 | 5.760 × 10−5 | 1 | 5.760 × 10−5 | 0.43 | 0.532 | |
| Residual | 9.308 × 10−4 | 7 | 1.330 × 10−4 | |||
| Lack of Fit | 3.488 × 10−4 | 3 | 1.163 × 10−4 | 0.80 | 0.556 | not significant |
| Pure Error | 5.820 × 10−4 | 4 | 1.455 × 10−4 | |||
| Cor Total | 1.525 × 10−3 | 16 |
| RSB (g) | TDI (g) | Glycerin (g) | Adsorption Capacity (Predicted) (mg/g) | Desirability | Adsorption Capacity (Actual) (mg/g) |
|---|---|---|---|---|---|
| 0.15 | 3.0 | 0.4 | 0.224 | 0.820 | 0.226 |
| Isotherm Models | Parameters | Value |
|---|---|---|
| Langmuir | Adjusted-R-Square | 0.902 |
| R-Square (COD) | 0.926 | |
| Reduced Chi-Sqr | 5.01 × 10−4 | |
| Kl | 0.505 ± 0.09597 | |
| Qm | 1.21478 ± 0.02203 | |
| Freundlich | Adjusted-R-Square | 0.970 |
| R-Square (COD) | 0.977 | |
| Reduced Chi-Sqr | 1.54 × 10−4 | |
| Kf | 0.79976 ± 0.02405 | |
| n | 9.74569 ± 0.88071 |
| Kinetics Models | Parameters | Concentration (ppm) | ||||
|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | ||
| Pseudo order I | Adjusted-R2 | 0.951 | 0.978 | 0.787 | 0.929 | 0.970 |
| K (g/mg.min) | 0.016 | 0.012 | 0.014 | 0.011 | 0.020 | |
| Qe (mg/g) | 0.640 | 0.620 | 0.664 | 0.630 | 0.565 | |
| Pseudo order II | Adjusted-R2 | 0.997 | 0.994 | 0.989 | 0.990 | 0.999 |
| K (g/mg.min) | 0.118 | 0.129 | 0.1378 | 0.109 | 0.192 | |
| Qe (mg/g) | 1.025 | 1.060 | 1.096 | 1.155 | 1.194 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurman, S.; Saiful; Rahmi; Ginting, B.; Marlina. Red Seaweed (Gracilaria verrucosa Greville) Based Polyurethane as Adsorptive Membrane for Ammonia Removal in Water. Polymers 2022, 14, 1572. https://doi.org/10.3390/polym14081572
Nurman S, Saiful, Rahmi, Ginting B, Marlina. Red Seaweed (Gracilaria verrucosa Greville) Based Polyurethane as Adsorptive Membrane for Ammonia Removal in Water. Polymers. 2022; 14(8):1572. https://doi.org/10.3390/polym14081572
Chicago/Turabian StyleNurman, Salfauqi, Saiful, Rahmi, Binawati Ginting, and Marlina. 2022. "Red Seaweed (Gracilaria verrucosa Greville) Based Polyurethane as Adsorptive Membrane for Ammonia Removal in Water" Polymers 14, no. 8: 1572. https://doi.org/10.3390/polym14081572