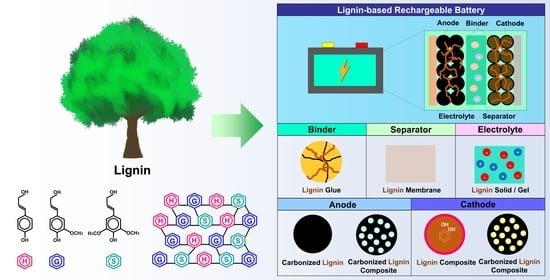
Lignin-Based Materials for Sustainable Rechargeable Batteries
Abstract
:1. Introduction
2. Lignin-Based Binders, Separators, and Electrolytes
2.1. Lignin-Based Binder
2.2. Lignin-Based Separator
2.3. Lignin-Based Electrolyte
3. Lignin-Based Anodes
4. Lignin-Based Cathodes
5. Challenges and Outlook
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Mohan, D.; Teong, Z.K.; Bakir, A.N.; Sajab, M.S.; Kaco, H. Extending cellulose-based polymers application in additive manufacturing technology: A review of recent approaches. Polymers 2020, 12. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, Z.; Xiang, Y.; Liu, D.; Xie, Z.; Qu, D.; Sun, M.; Tang, H.; Li, J. Cellulose-based material in lithium-sulfur batteries: A review. Carbohydr. Polym. 2011, 255, 117469. [Google Scholar] [CrossRef]
- Kumar, S.; Mohanty, A.; Erickson, L.; Misra, M. Lignin and its applications with polymers. J. Biobased Mater. Bioenergy 2019, 3, 1–24. [Google Scholar] [CrossRef]
- Gharehkhani, S.; Zhang, Y.; Fatehi, P. Lignin-derived platform molecules through tempo catalytic oxidation strategies. Prog. Energy Combust. Sci. 2019, 72, 59–89. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Y.; Qian, Y.; Qiu, X.; Ren, Y.; Yang, D. Reduction of lignin color via one-step uv irradiation. Green Chem. 2016, 18, 695–699. [Google Scholar] [CrossRef]
- Qian, Y.; Deng, Y.; Qiu, X.; Li, H.; Yang, D. Formation of uniform colloidal spheres from lignin, a renewable resource recovered from pulping spent liquor. Green Chem. 2014, 16, 2156–2163. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Yang, N.W.; Wang, Y.G. Preparation and evaluation of sodium hydroxymethyl lignosulfonate as eco-friendly drilling fluid additive. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2012; Volume 415, pp. 629–632. [Google Scholar]
- Corey, A.; Wamsley, K.; Winowiski, T.; Moritz, J. Effects of calcium lignosulfonate, mixer-added fat, and feed form on feed manufacture and broiler performance. J. Appl. Poult. Res. 2014, 23, 418–428. [Google Scholar] [CrossRef]
- Hemmilä, V.; Adamopoulos, S.; Hosseinpourpia, R.; Ahmed, S.A. Ammonium lignosulfonate adhesives for particleboards with pmdi and furfuryl alcohol as crosslinkers. Polymers 2019, 11, 1633. [Google Scholar] [CrossRef] [Green Version]
- Brethauer, S.; Shahab, R.L.; Studer, M.H. Impacts of biofilms on the conversion of cellulose. Appl. Microbiol. Biotechnol. 2020, 104, 5201–5212. [Google Scholar] [CrossRef]
- Wakerley, D.W.; Kuehnel, M.F.; Orchard, K.L.; Ly, K.H.; Rosser, T.E.; Reisner, E. Solar-driven reforming of lignocellulose to h2 with a cds/cdox photocatalyst. Nat. Energy 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Davin, L.B.; Lewis, N.G. Lignin primary structures and dirigent sites. Curr. Opin. Biotechnol. 2005, 16, 407–415. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, C.; Zhang, X.; Guo, B. Implementation and evaluation of a practical electrochemical- thermal model of lithium-ion batteries for ev battery management system. Energy 2021, 221. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief history of early lithium-battery development. Materials 2020, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nature Energy 2016, 1. [Google Scholar] [CrossRef]
- Choi, J.; Kim, P.J. A roadmap of battery separator development: Past and future. Curr. Opin. Electrochem. 2022, 31. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.-y.; He, Z.-j.; Li, Y.-j.; Mao, J.; Dai, K.-h.; Yan, C.; Zheng, J.-c. An advance review of solid-state battery: Challenges, progress and prospects. Sustain. Mater. Technol. 2021, 29. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, K. Building better batteries in the solid state: A review. Materials 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Dong, H.; Aurbach, D.; Yao, Y. Current status and future directions of multivalent metal-ion batteries. Nat. Energy 2020, 5, 646–656. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Ye, L.; Li, X. A dynamic stability design strategy for lithium metal solid state batteries. Nature 2021, 593, 218–222. [Google Scholar] [CrossRef]
- Deng, W.; Liang, X.; Wu, X.; Qian, J.; Cao, Y.; Ai, X.; Feng, J.; Yang, H. A low cost, all-organic na-ion battery based on polymeric cathode and anode. Sci. Rep. 2013, 3, 2671. [Google Scholar] [CrossRef]
- Deng, L.; Qu, J.; Niu, X.; Liu, J.; Zhang, J.; Hong, Y.; Feng, M.; Wang, J.; Hu, M.; Zeng, L.; et al. Defect-free potassium manganese hexacyanoferrate cathode material for high-performance potassium-ion batteries. Nat. Commun. 2021, 12, 2167. [Google Scholar] [CrossRef]
- Bi, S.; Wang, S.; Yue, F.; Tie, Z.; Niu, Z. A rechargeable aqueous manganese-ion battery based on intercalation chemistry. Nat. Commun. 2021, 12, 6991. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, C.; Zhang, S.; Song, X.; Tang, Y.; Cheng, H.-M. Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat. Chem. 2018, 10, 667–672. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Wei, C.-Y.; Lin, M.-C.; Pan, C.-J.; Chou, H.-L.; Chen, H.-A.; Gong, M.; Wu, Y.; Yuan, C.; Angell, M.; et al. Advanced rechargeable aluminium ion battery with a high-quality natural graphite cathode. Nat. Commun. 2017, 8, 14283. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From lithium-ion to sodium-ion batteries: Advantages, challenges, and surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, J.; Wang, C.; Liu, J.; Pu, Y.; Ragauskas, A.; Li, S.; Yang, B. Lignin-derived electrochemical energy materials and systems. Biofuels Bioprod. Biorefining 2020, 14, 650–672. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, J.; Chong, C.; Yu, X.; Wang, L.; Shi, Z. An electrospun lignin/polyacrylonitrile nonwoven composite separator with high porosity and thermal stability for lithium-ion batteries. RSC Adv. 2015, 5, 101115–101120. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, K.; Ma, J.; Xu, G.; Dong, S.; Chen, B.; Li, J.; Chen, Z.; Zhou, X.; Cui, G. A biomass based free radical scavenger binder endowing a compatible cathode interface for 5 v lithium-ion batteries. Energy Environ. Sci. 2019, 12, 273–280. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Y.; Tan, H.; Zhang, B.J.C. Advanced lignin-derived hard carbon for na-ion batteries and a comparison with li and k ion storage. Carbon 2020, 157, 316–323. [Google Scholar] [CrossRef]
- Nirmale, T.C.; Kale, B.B.; Varma, A.J. A review on cellulose and lignin based binders and electrodes: Small steps towards a sustainable lithium ion battery. Int. J. Biol. Macromol. 2017, 103, 1032–1043. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Hatakeyama, T. Lignin structure, properties, and applications. In Biopolymers; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–63. [Google Scholar]
- Pu, Y.; Cao, S.; Ragauskas, A.J. Application of quantitative 31p nmr in biomass lignin and biofuel precursors characterization. Energy Environ. Sci. 2011, 4, 3154–3166. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Banu, J.R.; Saratale, R.G.; Chang, S.W.; Kumar, G. A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour. Technol. 2019, 271, 462–472. [Google Scholar] [CrossRef]
- Uddin, M.-J.; Alaboina, P.K.; Zhang, L.; Cho, S.-J. A low-cost, environment-friendly lignin-polyvinyl alcohol nanofiber separator using a water-based method for safer and faster lithium-ion batteries. Mater. Sci. Eng. B 2017, 223, 84–90. [Google Scholar] [CrossRef]
- Lu, H.; Cornell, A.; Alvarado, F.; Behm, M.; Leijonmarck, S.; Li, J.; Tomani, P.; Lindbergh, G. Lignin as a binder material for eco-friendly li-ion batteries. Materials 2016, 9, 127. [Google Scholar] [CrossRef] [Green Version]
- Shabanov, N.S.; Rabadanov, K.S.; Gafurov, M.M.; Isaev, A.B.; Sobola, D.S.; Suleimanov, S.I.; Amirov, A.M.; Asvarov, A.S. Lignin-based gel polymer electrolyte for cationic conductivity. Polymers 2021, 13, 2306. [Google Scholar] [CrossRef]
- Yen, J.-P.; Chang, C.-C.; Lin, Y.-R.; Shen, S.-T.; Hong, L.J. Effects of styrene-butadiene rubber/carboxymethylcellulose (sbr/cmc) and polyvinylidene difluoride (pvdf) binders on low temperature lithium ion batteries. J. Electrochem. Soc. 2013, 160, A1811–A1818. [Google Scholar] [CrossRef]
- Dominguez-Robles, J.; Sanchez, R.; Diaz-Carrasco, P.; Espinosa, E.; Garcia-Dominguez, T.M.; Rodriguez, A. Isolation and characterization of lignins from wheat straw: Application as binder in lithium batteries. Int. J. Biol. Macromol. 2017, 104, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Domi, Y.; Usui, H.; Yamaguchi, K.; Yodoya, S.; Sakaguchi, H. Silicon-based anodes with long cycle life for lithium-ion batteries achieved by significant suppression of their volume expansion in ionic-liquid electrolyte. ACS Appl. Mater. Interfaces 2019, 11, 2950–2960. [Google Scholar] [CrossRef]
- Luo, C.; Du, L.; Wu, W.; Xu, H.; Zhang, G.; Li, S.; Wang, C.; Lu, Z.; Deng, Y. Novel lignin-derived water-soluble binder for micro silicon anode in lithium-ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 12621–12629. [Google Scholar] [CrossRef]
- Hassoun, J.; Derrien, G.; Panero, S.; Scrosati, B. A nanostructured sn-c composite lithium battery electrode with unique stability and high electrochemical performance. Adv. Mater. 2008, 20, 3169–3175. [Google Scholar] [CrossRef]
- Huang, X. Separator technologies for lithium-ion batteries. J. Solid State Electrochem. 2010, 15, 649–662. [Google Scholar] [CrossRef]
- Li, W.; Yao, H.; Yan, K.; Zheng, G.; Liang, Z.; Chiang, Y.M.; Cui, Y. The synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth. Nat. Commun. 2015, 6, 7436. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, H.; Du, Y.; Zhang, J. Semi-liquid anode for dendrite-free k-ion and na-ion batteries. Chem. Eng. J. 2021, 412, 128597. [Google Scholar] [CrossRef]
- Lee, B.; Paek, E.; Mitlin, D.; Lee, S.W. Sodium metal anodes: Emerging solutions to dendrite growth. Chem. Rev. 2019, 119, 5416–5460. [Google Scholar] [CrossRef]
- Tao, L.; Xu, Z.; Kuai, C.; Zheng, X.; Wall, C.E.; Jiang, C.; Esker, A.R.; Zheng, Z.; Lin, F. Flexible lignin carbon membranes with surface ozonolysis to host lean lithium metal anodes for nickel-rich layered oxide batteries. Energy Storage Mater. 2020, 24, 129–137. [Google Scholar] [CrossRef]
- Wang, Y.; Travas-Sejdic, J.; Steiner, R. Polymer gel electrolyte supported with microporous polyolefin membranes for lithium ion polymer battery. Solid State Ion. 2002, 148, 443–449. [Google Scholar] [CrossRef]
- Jeong, D.; Shim, J.; Shin, H.; Lee, J.C. Sustainable lignin-derived cross-linked graft polymers as electrolyte and binder materials for lithium metal batteries. ChemSusChem 2020, 13, 2642–2649. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, S.; Wei, Y.; Bian, H.; Wang, R.; Min, Y. Lignin nanoparticle-coated celgard separator for high-performance lithium-sulfur batteries. Polymers 2019, 11, 1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Advances in lithium–sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 2016, 1, 16132. [Google Scholar] [CrossRef]
- Aurbach, D.; Talyosef, Y.; Markovsky, B.; Markevich, E.; Zinigrad, E.; Asraf, L.; Gnanaraj, J.S.; Kim, H.-J. Design of electrolyte solutions for li and li-ion batteries: A review. Electrochim. Acta 2004, 50, 247–254. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, J.; Wang, Y.; Song, M.; Long, L.; Siyal, S.H.; Yang, X.; Sui, G. Recent advances in gel polymer electrolyte for high-performance lithium batteries. J. Energy Chem. 2019, 37, 126–142. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zhou, D.; He, Y.-B.; Fu, Y.; Qin, X.; Miao, C.; Du, H.; Li, B.; Yang, Q.-H.; Lin, Z.; et al. Novel gel polymer electrolyte for high-performance lithium–sulfur batteries. Nano Energy 2016, 22, 278–289. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and interphases in li-ion batteries and beyond. Chem Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Hou, T.-Z.; Zhang, R.; Peng, H.-J.; Zhao, C.-Z.; Huang, J.-Q.; Zhang, Q. Dendrite-free lithium deposition induced by uniformly distributed lithium ions for efficient lithium metal batteries. Adv. Mater. 2016, 28, 2888–2895. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Liang, Z.; Zhao, J.; Yan, K.; Cui, Y. Lithium-coated polymeric matrix as a minimum volume-change and dendrite-free lithium metal anode. Nat. Commun. 2016, 7, 10992. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Yin, X.; Kang, S.; Chen, Z.; Tian, B.; Teo, S.L.; Wang, X.; Chi, X.; Loh, K.P.; Lee, H.-W.; et al. Lithium silicide surface enrichment: A solution to lithium metal battery. Adv. Mater. 2018, 30, 1801745. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Nai, J.; Wang, Y.; Liu, T.; Zheng, J.; Yuan, H.; Sheng, O.; Jin, C.; Zhang, W.; Jin, Z.; et al. Biomacromolecules enabled dendrite-free lithium metal battery and its origin revealed by cryo-electron microscopy. Nat. Commun. 2020, 11, 488. [Google Scholar] [CrossRef] [Green Version]
- Cao, D.; Zhang, Q.; Hafez, A.M.; Jiao, Y.; Ma, Y.; Li, H.; Cheng, Z.; Niu, C.; Zhu, H. Lignin-derived holey, layered, and thermally conductive 3d scaffold for lithium dendrite suppression. Small Methods 2019, 3, 1800539. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Y.; Cao, H.; Song, A.; Lin, Y.; Wang, M.; Li, X. A high-performance and environment-friendly gel polymer electrolyte for lithium ion battery based on composited lignin membrane. J. Solid State Electrochem. 2017, 22, 807–816. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Wang, A.; Liu, X.; Chen, J.; Wang, Z.; Zeng, Q.; Zhou, H.-h.; Jiang, X.; Zhang, L. Polymer-laden composite lignin-based electrolyte membrane for high-performance lithium batteries. ACS Sustain. Chem. Eng. 2018, 6, 14460–14469. [Google Scholar] [CrossRef]
- Xu, J.; Dou, Y.; Wei, Z.; Ma, J.; Deng, Y.; Li, Y.; Liu, H.; Dou, S. Recent progress in graphite intercalation compounds for rechargeable metal (li, na, k, al)-ion batteries. Adv. Sci. 2017, 4, 1700146. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Lu, H.; Fang, Y.; Sushko, M.L.; Cao, Y.; Ai, X.; Yang, H.; Liu, J. Low-defect and low-porosity hard carbon with high coulombic efficiency and high capacity for practical sodium ion battery anode. Adv. Energy Mater. 2018, 8, 1703238. [Google Scholar] [CrossRef]
- Hong, K.L.; Qie, L.; Zeng, R.; Yi, Z.Q.; Zhang, W.; Wang, D.; Yin, W.; Wu, C.; Fan, Q.J.; Zhang, W.X.; et al. Biomass derived hard carbon used as a high performance anode material for sodium ion batteries. J. Mater. Chem. A 2014, 2, 12733–12738. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, X.; Wang, Q.; Xu, X.; Zhou, X.; Bao, J. Kelp-derived hard carbons as advanced anode materials for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 5761–5769. [Google Scholar] [CrossRef]
- Zhai, S.; Jiang, W.; Wei, L.; Karahan, H.E.; Yuan, Y.; Ng, A.K.; Chen, Y. All-carbon solid-state yarn supercapacitors from activated carbon and carbon fibers for smart textiles. Mater. Horiz. 2015, 2, 598–605. [Google Scholar] [CrossRef]
- Dorrestijn, E.; Laarhoven, L.J.; Arends, I.W.; Mulder, P. The occurrence and reactivity of phenoxyl linkages in lignin and low rank coal. J. Anal. Appl. Pyrolysis 2000, 54, 153–192. [Google Scholar] [CrossRef]
- Varhegyi, G.; Antal, M.J., Jr.; Jakab, E.; Szabó, P. Kinetic modeling of biomass pyrolysis. J. Anal. Appl. Pyrolysis 1997, 42, 73–87. [Google Scholar] [CrossRef] [Green Version]
- Iatridis, B.; Gavalas, G.R. Pyrolysis of a precipitated kraft lignin. Ind. Eng. Chem. Prod. Res. Dev. 1997, 18, 127–130. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in green polymer composites from lignin for multifunctional applications: A review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Chatterjee, S.; Saito, T. Lignin-derived advanced carbon materials. ChemSusChem 2015, 8, 3941–3958. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, T.; Zhang, W.X.; Chen, S.; Mezgebe, M. Lignin-based activated carbon fibers and controllable pore size and properties. Appl. Polym. Sci. 2011, 121, 989–994. [Google Scholar] [CrossRef]
- Wang, S.X.; Yang, L.; Stubbs, L.P.; Li, X.; He, C. Lignin-derived fused electrospun carbon fibrous mats as high performance anode materials for lithium ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 12275–12282. [Google Scholar] [CrossRef]
- Yoon, D.; Hwang, J.; Chang, W.; Kim, J. Carbon with expanded and well-developed graphene planes derived directly from condensed lignin as a high-performance anode for sodium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 569–581. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Zhu, M.; Wang, W.; Wang, L.; Xie, S.; Wang, L.; Yang, X.; He, X.; Sun, Y. Recycling of lignin and si waste for advanced si/c battery anodes. ACS Appl. Mater. Interfaces 2020, 12, 57055–57063. [Google Scholar] [CrossRef]
- Xi, Y.; Yang, D.; Lou, H.; Gong, Y.; Yi, C.; Lyu, G.; Han, W.; Kong, F.; Qiu, X. Designing the effective microstructure of lignin-based porous carbon substrate to inhibit the capacity decline for sno2 anode. Ind. Crops Prod. 2021, 161, 113179. [Google Scholar] [CrossRef]
- Jia, H.; Sun, N.; Dirican, M.; Li, Y.; Chen, C.; Zhu, P.; Yan, C.; Zang, J.; Guo, J.; Tao, J.; et al. Electrospun kraft lignin/cellulose acetate-derived nanocarbon network as an anode for high-performance sodium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 44368–44375. [Google Scholar] [CrossRef]
- Matei Ghimbeu, C.; Zhang, B.; de Yuso, A.M.; Réty, B.; Tarascon, J.-M. Valorizing low cost and renewable lignin as hard carbon for na-ion batteries: Impact of lignin grade. Carbon 2019, 153, 634–647. [Google Scholar] [CrossRef]
- Dizhbite, T.; Zakis, G.; Kizima, A.; Lazareva, E.; Rossinskaya, G.; Jurkjane, V.; Telysheva, G.; Viesturs, U. Lignin—A useful bioresource for the production of sorption-active materials. Bioresour. Technol. 1999, 67, 221–228. [Google Scholar] [CrossRef]
- Li, C.; Sun, Y.; Wu, Q.; Liang, X.; Chen, C.; Xiang, H. A novel design strategy of a practical carbon anode material from a single lignin-based surfactant source for sodium-ion batteries. Chem. Commun. 2020, 56, 6078–6081. [Google Scholar] [CrossRef]
- Wu, Z.; Zou, J.; Zhang, Y.; Lin, X.; Fry, D.; Wang, L.; Liu, J. Lignin-derived hard carbon anode for potassium-ion batteries: Interplay among lignin molecular weight, material structures, and storage mechanisms. Chem. Eng. J. 2022, 427, 131547. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, L.; Toprakci, O.; Liang, Y.; Alcoutlabi, M. Electrospun nanofiber-based anodes, cathodes, and separators for advanced lithium-ion batteries. Polym. Rev. 2011, 51, 239–264. [Google Scholar] [CrossRef]
- Chung, D. Carbon Fiber Composites; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Huang, X. Fabrication and properties of carbon fibers. Materials 2009, 2, 2369–2403. [Google Scholar] [CrossRef]
- Dallmeyer, I.; Ko, F.; Kadla, J.F. Correlation of elongational fluid properties to fiber diameter in electrospinning of softwood kraft lignin solutions. Ind. Eng. Chem. Res. 2014, 53, 2697–2705. [Google Scholar] [CrossRef]
- Hao, X.; Zeng, Y. A review on the studies of air flow field and fiber formation process during melt blowing. Ind. Eng. Chem. Res. 2019, 58, 11624–11637. [Google Scholar] [CrossRef]
- Wu, Y.; Fang, S.; Jiang, Y. Carbon anode materials based on melamine resin. Mater. Chem. 1998, 8, 2223–2227. [Google Scholar] [CrossRef]
- Wu, M.; Xiao, X.; Vukmirovic, N.; Xun, S.; Das, P.K.; Song, X.; Olalde-Velasco, P.; Wang, D.; Weber, A.Z.; Wang, L.W.; et al. Toward an ideal polymer binder design for high-capacity battery anodes. J. Am. Chem. Soc. 2013, 135, 12048–12056. [Google Scholar] [CrossRef] [Green Version]
- Way, B.; Dahn, J. The effect of boron substitution in carbon on the intercalation of lithium in li x (b z c 1− z) 6. Electrochem. Soc. 1994, 141, 907. [Google Scholar] [CrossRef]
- Fan, L.; Shi, Z.; Ren, Q.; Yan, L.; Zhang, F.; Fan, L. Nitrogen-doped lignin based carbon microspheres as anode material for high performance sodium ion batteries. Green Energy Environ. 2021, 6, 220–228. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, J.; Lin, Z.; Lin, H.; Lu, H.; Wang, Y.; Huang, W. Facile preparation of 3d hierarchical porous carbon from lignin for the anode material in lithium ion battery with high rate performance. Electrochimica Acta 2015, 176, 1136–1142. [Google Scholar] [CrossRef]
- Heiska, J.; Nisula, M.; Karppinen, M. Organic electrode materials with solid-state battery technology. J. Mater. Chem. A 2019, 7, 18735–18758. [Google Scholar] [CrossRef] [Green Version]
- Mauger, A.; Julien, C.; Paolella, A.; Armand, M.; Zaghib, K. Recent progress on organic electrodes materials for rechargeable batteries and supercapacitors. Materials 2019, 12, 1770. [Google Scholar] [CrossRef] [Green Version]
- Hanyu, Y.; Honma, I. Rechargeable quasi-solid state lithium battery with organic crystalline cathode. Sci. Rep. 2012, 2, 453. [Google Scholar] [CrossRef] [Green Version]
- Ajjan, F.; Casado, N.; Rębiś, T.; Elfwing, A.; Solin, N.; Mecerreyes, D.; Inganäs, O. High performance pedot/lignin biopolymer composites for electrochemical supercapacitors. J. Mater. Chem. A 2016, 4, 1838–1847. [Google Scholar] [CrossRef] [Green Version]
- Ajjan, F.N.; Vagin, M.; Rębiś, T.; Aguirre, L.E.; Ouyang, L.; Inganäs, O. Scalable asymmetric supercapacitors based on hybrid organic/biopolymer electrodes. Adv. Sustain. Syst. 2017, 1, 1700054. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, Y.; Jiao, L.; Yang, L.; Hamel, J.; Giummarella, N.; Henriksson, G.; Zhang, L.; Zhu, H. Bioinspired ultrastable lignin cathode via graphene reconfiguration for energy storage. ACS Sustain. Chem. Eng. 2017, 5, 3553–3561. [Google Scholar] [CrossRef]
- Casado, N.; Hilder, M.; Pozo-Gonzalo, C.; Forsyth, M.; Mecerreyes, D. Electrochemical behavior of pedot/lignin in ionic liquid electrolytes: Suitable cathode/electrolyte system for sodium batteries. ChemSusChem 2017, 10, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Suárez, A.; Carretero-González, J.; Casado, N.; Mecerreyes, D.; Rojo, T.; Castillo-Martinez, E. Hybrid biopolymer electrodes for lithium-and sodium-ion batteries in organic electrolytes. Sustain. Energy 2018, 2, 836–842. [Google Scholar] [CrossRef]
- Lahiri, A.; Yang, L.; Höfft, O.; Endres, F. Biodegradable zn-ion battery with a lignin composite electrode and bio-ionic liquid based electrolyte: Possible in situ energy generation by lignin electrocatalysis. Mater. Adv. 2021, 2, 2676–2683. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, A.; Huang, A.; Chen, M.; Tian, Q.; Chen, J.; Xu, X. Hybridizing δ-type mno2 with lignin-derived porous carbon as a stable cathode material for aqueous zn–mno2 batteries. Front. Energy Res. 2020, 182. [Google Scholar] [CrossRef]
- Xu, J.; Hu, X.; Alam, M.A.; Muhammad, G.; Lv, Y.; Wang, M.; Zhu, C.; Xiong, W. Al-doped α-mno 2 coated by lignin for high-performance rechargeable aqueous zinc-ion batteries. RSC Adv. 2021, 11, 35280–35286. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Du, H.; Kang, F. Energetic zinc ion chemistry: The rechargeable zinc ion battery. Angew. Chem. 2012, 124, 957–959. [Google Scholar] [CrossRef]
- Xue, T.; Fan, H.J. From aqueous zn-ion battery to zn-mno2 flow battery: A brief story. J. Energy Chem. 2021, 54, 194–201. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Huang, Y.; Jiang, B.; Chang, Z.; Xu, C.; Kang, F. Β-mno2 with proton conversion mechanism in rechargeable zinc ion battery. J. Energy Chem. 2021, 56, 365–373. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Liu, S.; Li, P.; Yuan, D.; He, C. Hierarchical porous carbon monolith derived from lignin for high areal capacitance supercapacitors. Microporous Mesoporous Mater. 2020, 297, 109960. [Google Scholar] [CrossRef]
- Xia, L.; Zhou, Y.; Ren, J.; Wu, H.; Lin, D.; Xie, F.; Jie, W.; Lam, K.H.; Xu, C.; Zheng, Q. An eco-friendly microorganism method to activate biomass for cathode materials for high-performance lithium–sulfur batteries. Energy Fuels 2018, 32, 9997–10007. [Google Scholar] [CrossRef]
- Lu, P.; Liu, F.; Zhou, F.; Qin, J.; Shi, H.; Wu, Z.-S. Lignin derived hierarchical porous carbon with extremely suppressed polyselenide shuttling for high-capacity and long-cycle-life lithium–selenium batteries. J. Energy Chem. 2021, 55, 476–483. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Zhang, S.; Hou, Y. Sulfur hosts against the shuttle effect. Small Methods 2018, 2, 1700345. [Google Scholar] [CrossRef]
- Zhao, S.; Li, C.; Wang, W.; Zhang, H.; Gao, M.; Xiong, X.; Wang, A.; Yuan, K.; Huang, Y.; Wang, F. A novel porous nanocomposite of sulfur/carbon obtained from fish scales for lithium–sulfur batteries. J. Mater. Chem. A 2013, 1, 3334–3339. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, J.; Kim, S.-W.; Halim, W.; Frey, M.W.; Joo, Y.L. Effective suppression of the polysulfide shuttle effect in lithium–sulfur batteries by implementing rgo–pedot: Pss-coated separators via air-controlled electrospray. ACS Omega 3 2018, 3, 16465–16471. [Google Scholar] [CrossRef]
- Jin, C.; Sheng, O.; Zhang, W.; Luo, J.; Yuan, H.; Yang, T.; Huang, H.; Gan, Y.; Xia, Y.; Liang, C. Sustainable, inexpensive, naturally multi-functionalized biomass carbon for both li metal anode and sulfur cathode. Energy Storage Mater. 2018, 15, 218–225. [Google Scholar] [CrossRef]
- Babu, D.B.; Ramesha, K. Constraining polyselenide formation in ether based electrolytes through confinement of se in microporous carbon matrix for li-se batteries. Electrochim. Acta 2016, 219, 295–304. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, W.; Zhao, P.; Tao, L.; Liu, Y.; Manaig, D.; Freschi, D.J.; Liu, J. The role of carbon pore structure in tellurium/carbon cathodes for lithium-tellurium batteries. Electrochim. Acta 2021, 388, 138621. [Google Scholar] [CrossRef]
- Lai, Y.H.; Kuo, Y.T.; Lai, B.Y.; Lee, Y.C.; Chen, H.Y. Improving lithium-sulfur battery performance with lignin reinforced mwcnt protection layer. Int. J. Energy Res. 2019, 43, 5803–5811. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Wang, X.; Liu, G.; Li, J.; Zhao, R.; Zhang, Y.; Zhang, X.; Han, G.; Zhao, H. In-situ growth of graphene on carbon nanofiber from lignin. Carbon 2020, 169, 446–454. [Google Scholar] [CrossRef]
- Liu, T.; Sun, S.; Song, W.; Sun, X.; Niu, Q.; Liu, H.; Ohsaka, T.; Wu, J. A lightweight and binder-free electrode enabled by lignin fibers@ carbon-nanotubes and graphene for ultrastable lithium-sulfur batteries. J. Mater. Chem. A 2018, 6, 23486–23494. [Google Scholar] [CrossRef]
- Xu, J.; Liu, B.; Wu, L.; Hu, J.; Hou, H.; Yang, J. A waste-minimized biorefinery scenario for the hierarchical conversion of agricultural straw into prebiotic xylooligosaccharides, fermentable sugars and lithium-sulfur batteries. Ind. Crops 2019, 129, 269–280. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, P.; Yuan, L.; Liu, X.; Ma, J.; Zhang, C. Dual lignin valorization enabled by carbon quantum dots and lithium-sulfur cathode. Ind. Crops 2021, 170, 113801. [Google Scholar] [CrossRef]
- Yeon, J.S.; Park, S.H.; Suk, J.; Lee, H.; Park, H.S. Confinement of sulfur in the micropores of honeycomb-like carbon derived from lignin for lithium-sulfur battery cathode. Chem. Eng. J. 2020, 382, 122946. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Jia, M.; Nan, T.; Zhang, H.; Zhao, S.; Shen, Q. Elaborate construction and electrochemical properties of lignin-derived macro-/micro-porous carbon-sulfur composites for rechargeable lithium-sulfur batteries: The effect of sulfur-loading time. J. Alloy. 2017, 709, 677–685. [Google Scholar] [CrossRef]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.Y.; Lee, J.S.; Han, H.T.; Jung, J.; Eom, K.; Lee, J.T. Lignin-Based Materials for Sustainable Rechargeable Batteries. Polymers 2022, 14, 673. https://doi.org/10.3390/polym14040673
Jung HY, Lee JS, Han HT, Jung J, Eom K, Lee JT. Lignin-Based Materials for Sustainable Rechargeable Batteries. Polymers. 2022; 14(4):673. https://doi.org/10.3390/polym14040673
Chicago/Turabian StyleJung, Han Young, Jeong Seok Lee, Hyun Taek Han, Jaehan Jung, KwangSup Eom, and Jung Tae Lee. 2022. "Lignin-Based Materials for Sustainable Rechargeable Batteries" Polymers 14, no. 4: 673. https://doi.org/10.3390/polym14040673