A Critical Review on the Economically Feasible and Sustainable Poly(3-Hydroxybutyrate-co-3-hydroxyvalerate) Production from Alkyl Alcohols
Abstract
:1. General Overview
2. P(3HB-co-3HV) Properties and Applications
3. Bioconversion of Alkyl Alcohols and Organic Acids into P(3HB-co-3HV)
4. Techno-Economic and Sustainability Assessment
5. Oxo Synthesis of Alkyl Alcohols
5.1. 1-Propanol
5.2. 1-Pentanol
6. Biosynthesis of 1-Propanol and 1-Pentanol by Wild-Type Bacteria
6.1. The Wood–Werkman Pathway in Propionibacteria
6.2. The Acrylate Pathway in Clostridium
6.3. The Carboxylate Reduction Pathway in Clostridium
7. Biosynthesis of 1-Propanol and 1-Pentanol by Genetic-Engineered E. coli
7.1. Co-Expression of the Citramalate and Threonine Pathway
7.2. Interactive Elongation Cycle of 2-Ketoacids
7.3. Extended Dissimilation of Succinate
7.4. Acquired Carboxylate Reduction Pathway
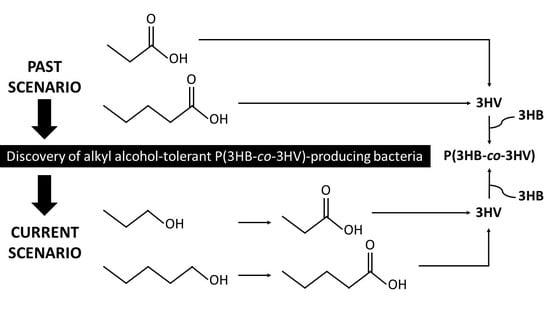
8. Alkyl Alcohol-Tolerant P(3HB-co-3HV)-Producing Bacteria
9. Mode of Action of 1-Propanol and 1-Pentanol on Proteins
10. Mechanisms Involved in Alcohols Tolerance
10.1. Changes in the Cell Membrane
10.2. Stress Response System
11. Challenges in Wide Implementation of Alkyl Alcohols as 3HV Precursors
12. Concluding Remark
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 3HB | 3-hydroxybutyrate |
| 3HV | 3-hydroxyvalerate |
| α-P(3HB) | Synthetic atactic poly(3-hydroxybutyrate) |
| Ag/BSA | Bovine serum albumin capped silver |
| AS | Ascorbic acid |
| CNC | Cellulose nanocrystals |
| CNT | Carbon nanotubes |
| DCP | Dicumyl peroxide |
| HA | Hydroxyapatite |
| HDPE | High-density polyethylene |
| LDPE | Low-density polyethylene |
| LLDPE | Linear low-density polyethylene |
| MAT | Organophilic attapulgite |
| mPEG | Monomethoxy poly(ethylene glycol) |
| NA | Not available |
| P(3HB) | Poly(3-hydroxybutyrate) |
| P(3HV) | Poly(3-hydroxyvalerate) |
| P(3HB-co-3HV) | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| PBAT | Poly(butylene adipate-co-terephthalate) |
| PBS | Poly(butylene succinate) |
| PCL | Poly(ε-caprolactone) |
| PDLLA | Poly(d,l-lactide) |
| PEG | Poly(ethylene glycol) |
| PHA | Polyhydroxyalkanoates |
| PHEMA | Poly(2-hydroxyl ethyl methacrylate) |
| PLA | Poly(lactic acid) |
| PP | Polypropylene |
| PPC | Poly(propylene carbonate) |
| Ref. | References |
| SBM | Sleeping beauty mutase |
References
- Pellis, A.; Malinconico, M.; Guarneri, A.; Gardossi, L. Renewable polymers and plastics: Performance beyond the green. New Biotechnol. 2020, 60, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Braunegg, G.; Lefebvre, G.; Genser, K.F. Polyhydroxyalkanoates, biopolyesters from renewable resources: Physiological and engineering aspects. J. Biotechnol. 1998, 65, 127–161. [Google Scholar] [CrossRef]
- Policastro, G.; Panico, A.; Fabbricino, M. Improving biological production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) co-polymer: A critical review. Rev. Environ. Sci. Biotechnol. 2021, 1–35. [Google Scholar] [CrossRef]
- Taguchi, S.; Iwata, T.; Abe, H.; Doi, Y. 9.09-Poly(hydroxyalkanoate)s. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 157–182. [Google Scholar] [CrossRef]
- Alvarez, H.M.; Kalscheuer, R.; Steinbüchel, A. Accumulation of storage lipids in species of Rhodococcus and Nocardia and effect of inhibitors and polyethylene glycol. Lipid/Fett 1997, 99, 239–246. [Google Scholar] [CrossRef]
- Anderson, A.J.; Williams, D.R.; Dawes, E.A.; Ewing, D.F. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in Rhodococcus ruber. Can. J. Microbiol. 1995, 41, 4–13. [Google Scholar] [CrossRef]
- Haywood, G.W.; Anderson, A.J.; Williams, D.R.; Dawes, E.A.; Ewing, D.F. Accumulation of a poly(hydroxyalkanoate) copolymer containing primarily 3-hydroxyvalerate from simple carbohydrate substrates by Rhodococcus sp. NCIMB 40126. Int. J. Biol. Macromol. 1991, 13, 83–88. [Google Scholar] [CrossRef]
- Valentin, H.F.; Dennis, D. Metabolic pathway for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) formation in Nocardia corallina: Inactivation of mutB by chromosomal integration of a kanamycin resistance gene. Appl. Environ. Microbiol. 1996, 62, 372–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, D.R.; Anderson, A.J.; Dawes, E.A.; Ewing, D.F. Production of a co-polyester of 3-hydroxybutyric acid and 3-hydroxyvaleric acid from succinic acid by Rhodococcus ruber: Biosynthetic considerations. Appl. Microbiol. Biotechnol. 1994, 40, 717–723. [Google Scholar] [CrossRef]
- Berezina, N. Enhancing the 3-hydroxyvalerate component in bioplastic PHBV production by Cupriavidus necator. Biotechnol. J. 2012, 7, 304–309. [Google Scholar] [CrossRef]
- Novackova, I.; Kucera, D.; Porizka, J.; Pernicova, I.; Sedlacek, P.; Koller, M.; Kovalcik, A.; Obruca, S. Adaptation of Cupriavidus necator to levulinic acid for enhanced production of P(3HB-co-3HV) copolyesters. Biochem. Eng. J. 2019, 151, 107350–107360. [Google Scholar] [CrossRef]
- Scully, S.M.; Orlygsson, J. Biological production of alcohols. In Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals, and Bioproducts; Woodhead Publishing: Cambridge, MA, USA, 2019; pp. 83–108. [Google Scholar]
- Obruca, S.; Marova, I.; Snajdar, O.; Mravcova, L.; Svoboda, Z. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Cupriavidus necator from waste rapeseed oil using propanol as a precursor of 3-hydroxyvalerate. Biotechnol. Lett. 2010, 32, 1925–1932. [Google Scholar] [CrossRef]
- Yamane, T.; Chen, X.; Ueda, S. Growth-associated production of poly(3-hydroxyvalerate) from n-pentanol by a methylotrophic bacterium, Paracoccus denitrificans. Appl. Environ. Microbiol. 1996, 62, 380–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strong, P.J.; Laycock, B.; Mahamud, S.N.S.; Jensen, P.D.; Lant, P.A.; Tyson, G.; Pratt, S. The opportunity for high-performance biomaterials from methane. Microorganisms 2016, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, H.; Deng, B.; Zhao, X. Poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate): Structure, property, and fiber. Int. J. Polym. Sci. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Kamendov, I.V.; Starosvetsky, S.I.; Vinnik, Y.S.; Markelova, N.N.; Shageev, A.A.; Khorzhevsky, V.A.; Peryanova, O.V.; Shumilova, A.A. An in vivo study of osteoplastic properties of resorbable poly-3-hydroxybutyrate in models of segmental osteotomy and chronic osteomyelitis. Artif. Cells, Nanomed. Biotechnol. 2014, 42, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Naser, A.Z.; Deiab, I.; Darras, B.M. Poly(lactic acid)(PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: A review. RSC Adv. 2021, 11, 17151–17196. [Google Scholar] [CrossRef]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Ali, I.; Jamil, N. Polyhydroxyalkanoates: Current applications in the medical field. Front. Biol. 2016, 11, 19–27. [Google Scholar] [CrossRef]
- Wu, L.P.; Wang, D.; Parhamifar, L.; Hall, A.; Chen, G.Q.; Moghimi, S.M. Poly(3-hydroxybutyrate-co-R-3-hydroxyhexanoate) nanoparticles with polyethylenimine coat as simple, safe, and versatile vehicles for cell targeting: Population characteristics, cell uptake, and intracellular trafficking. Adv. Healthc. Mater. 2014, 3, 817–824. [Google Scholar] [CrossRef]
- Catoni, S.E.; Trindade, K.N.; Gomes, C.A.; Schneider, A.L.; Pezzin, A.; Soldi, V. Influence of poly(ethylene grycol)-(PEG) on the properties of influence of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-PHBV. Polímeros 2013, 23, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Loh, X.J. Water soluble polyhydroxyalkanoates: Future materials for therapeutic applications. Chem. Soc. Rev. 2015, 44, 2865–2879. [Google Scholar] [CrossRef]
- Shah, M.; Naseer, M.I.; Choi, M.H.; Kim, M.O.; Yoon, S.C. Amphiphilic PHA–mPEG copolymeric nanocontainers for drug delivery: Preparation, characterization and in vitro evaluation. Int. J. Pharm. 2010, 400, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Scandola, M.; Focarete, M.L.; Adamus, G.; Sikorska, W.; Baranowska, I.; Świerczek, S.; Gnatowski, M.; Kowalczuk, M.; Jedliński, Z. Polymer blends of natural poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and a synthetic atactic poly(3-hydroxybutyrate). Characterization and biodegradation studies. Macromolecules 1997, 30, 2568–2574. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Wadhwa, P.; Hong, J.W.; Hong, Y.G.; Jeon, J.M.; Lee, E.S.; Yang, Y.H. Lipase mediated functionalization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with ascorbic acid into an antioxidant active biomaterial. Int. J. Biol. Macromol. 2019, 123, 117–123. [Google Scholar] [CrossRef]
- Malmir, S.; Montero, B.; Rico, M.; Barral, L.; Bouza, R. Morphology, thermal and barrier properties of biodegradable films of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) containing cellulose nanocrystals. Compos. A Appl. Sci. Manuf. 2017, 93, 41–48. [Google Scholar] [CrossRef]
- Meereboer, K.W.; Pal, A.K.; Cisneros-López, E.O.; Misra, M.; Mohanty, A.K. The effect of natural fillers on the marine biodegradation behaviour of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)(PHBV). Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Cabedo, L.; Lagaron, J.M.; Torres-Giner, S. Development of electrospun poly(3-hydroxybutyrate-co-3-hydroxyvalerate) monolayers containing eugenol and their application in multilayer antimicrobial food packaging. Front. Nutr. 2020, 7, 140–155. [Google Scholar] [CrossRef]
- Galego, N.; Rozsa, C.; Sánchez, R.; Fung, J.; Vázquez, A.; Santo Tomás, J. Characterization and application of poly(β-hydroxyalkanoates) family as composite biomaterials. Polym. Test. 2000, 19, 485–492. [Google Scholar] [CrossRef]
- Thiré, R.M.D.S.M.; Arruda, L.C.; Barreto, L.S. Morphology and thermal properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/attapulgite nanocomposites. Mater. Res. 2011, 14, 340–344. [Google Scholar] [CrossRef] [Green Version]
- Kwiecien, I.; Adamus, G.; Jiang, G.; Radecka, I.; Baldwin, T.C.; Khan, H.R.; Johnston, B.; Pennetta, V.; Hill, D.; Bretz, I.; et al. Biodegradable PBAT/PLA blend with bioactive MCPA-PHBV conjugate suppresses weed growth. Biomacromolecules 2018, 19, 511–520. [Google Scholar] [CrossRef]
- Bakare, R.A.; Bhan, C.; Raghavan, D. Synthesis and characterization of collagen grafted poly(hydroxybutyrate–valerate)(PHBV) scaffold for loading of bovine serum albumin capped silver (Ag/BSA) nanoparticles in the potential use of tissue engineering application. Biomacromolecules 2014, 15, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cornish, K.; Vodovotz, Y. Synergistic mechanisms underlie the peroxide and coagent improvement of natural-rubber-toughened poly(3-hydroxybutyrate-co-3-hydroxyvalerate) mechanical performance. Polymers 2019, 11, 565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javadi, A.; Kramschuster, A.J.; Pilla, S.; Lee, J.; Gong, S.; Turng, L.S. Processing and characterization of microcellular PHBV/PBAT blends. Poly. Eng. Sci. 2010, 50, 1440–1448. [Google Scholar] [CrossRef]
- Qiu, Z.; Ikehara, T.; Nishi, T. Miscibility and crystallization behaviour of biodegradable blends of two aliphatic polyesters. Poly(3-hydroxybutyrate-co-hydroxyvalerate) and poly(butylene succinate) blends. Polymer 2003, 44, 7519–7527. [Google Scholar] [CrossRef]
- Ma, P.; Hristova-Bogaerds, D.G.; Lemstra, P.J.; Zhang, Y.; Wang, S. Toughening of PHBV/PBS and PHB/PBS blends via in situ compatibilization using dicumyl peroxide as a free-radical grafting initiator. Macromol. Mater. Eng. 2012, 297, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Chun, Y.S.; Kim, W.N. Thermal properties of poly(hydroxybutyrate-co-hydroxyvalerate) and poly(ε-caprolactone) blends. Polymer 2000, 41, 2305–2308. [Google Scholar] [CrossRef]
- Wang, S.; Ma, P.; Wang, R.; Wang, S.; Zhang, Y.; Zhang, Y. Mechanical, thermal and degradation properties of poly(d,l-lactide)/poly(hydroxybutyrate-co-hydroxyvalerate)/poly(ethylene glycol) blend. Polym. Degrad. Stab. 2008, 93, 1364–1369. [Google Scholar] [CrossRef]
- Pillai, A.B.; Kumar, A.J.; Kumarapillai, H. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) in Bacillus aryabhattai and cytotoxicity evaluation of PHBV/poly(ethylene glycol) blends. 3 Biotech 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Silva, A.P.B.; Montagna, L.S.; Passador, F.R.; Rezende, M.C.; Lemes, A.P. Biodegradable nanocomposites based on PLA/PHBV blend reinforced with carbon nanotubes with potential for electrical and electromagnetic applications. Express Polym. Lett. 2021, 15, 1–12. [Google Scholar] [CrossRef]
- Zhao, H.; Cui, Z.; Wang, X.; Turng, L.S.; Peng, X. Processing and characterization of solid and microcellular poly(lactic acid)/polyhydroxybutyrate-valerate (PLA/PHBV) blends and PLA/PHBV/Clay nanocomposites. Compos. B Eng. 2013, 51, 79–91. [Google Scholar] [CrossRef]
- Tao, J.; Song, C.; Cao, M.; Hu, D.; Liu, L.; Liu, N.; Wang, S. Thermal properties and degradability of poly(propylene carbonate)/poly(β-hydroxybutyrate-co-β-hydroxyvalerate) (PPC/PHBV) blends. Polym. Degrad. Stab. 2009, 94, 575–583. [Google Scholar] [CrossRef]
- Syahirah, W.N.; Azami, N.A.; Huong, K.H.; Amirul, A.A. Preparation, characterization and biodegradation of blend films of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with natural biopolymers. Polym. Bull. 2020, 78, 3973–3993. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Fabra, M.J.; Pourrahimi, A.M.; Olsson, R.T.; Lagaron, J.M. The impact of zinc oxide particle morphology as an antimicrobial and when incorporated in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) films for food packaging and food contact surfaces applications. Food Bioprod. Process. 2017, 101, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Shuai, C.; Wang, C.; Qi, F.; Peng, S.; Yang, W.; He, C.; Wang, G.; Qian, G. Enhanced crystallinity and antibacterial of PHBV scaffolds incorporated with zinc oxide. J. Nanomater. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Alsabri, A.; Tahir, F.; Al-Ghamdi, S.G. Environmental impacts of polypropylene (PP) production and prospects of its recycling in the GCC region. Mat. Today Proc. 2021; in press. [Google Scholar] [CrossRef]
- Sen, S.K.; Raut, S. Microbial degradation of low density polyethylene (LDPE): A review. J. Environ. Chem. Eng. 2015, 3, 462–473. [Google Scholar] [CrossRef]
- Kader, M.A.; Senge, M.; Mojid, M.A.; Ito, K. Recent advances in mulching materials and methods for modifying soil environment. Soil Tillage Res. 2017, 168, 155–166. [Google Scholar] [CrossRef]
- Sarkar, D.J.; Barman, M.; Bera, T.; De, M.; Chatterjee, D. Agriculture: Polymers in crop production mulch and fertilizer. In Encyclopedia of Polymer Applications; Routledge: England, UK, 2018; Volume 1, pp. 1–20. [Google Scholar]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Yogesh, C.; Pathak, B.; Fulekar, M.H. PHA-production application and its bioremediation in environment. Res. J. Environ. Sci. 2012, 1, 46–52. Available online: http://www.isca.in/IJENS/Archive/v1/i2/9.ISCA-JEvsS-2012-009.pdf (accessed on 15 January 2022).
- Krasnits, E.; Beliavsky, M.; Tarre, S.; Green, M. PHA based denitrification: Municipal wastewater vs. acetate. Bioresour. Technol. 2013, 132, 28–37. [Google Scholar] [CrossRef]
- Santorio, S.; Fra-Vázquez, A.; Del Rio, A.V.; Mosquera-Corral, A. Potential of endogenous PHA as electron donor for denitrification. Sci. Total Environ. 2019, 695, 133747–133753. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, A.; Khan, S.T. Application of polyhydroxyalkanoates for denitrification in water and wastewater treatment. Appl. Microbiol. Biotechnol. 2003, 61, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H. Beta oxidation of fatty acids. Biochim. Biophys. Acta Lipids Lipid Metab. 1991, 1081, 109–120. [Google Scholar] [CrossRef]
- Azira, T.F.; Nursolehah, A.A.; Norhayati, Y.; Majid, M.I.A.; Amirul, A.A. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-4-hydroxybutyrate) terpolymer by Cupriavidus sp. USMAA2-4 through two-step cultivation process. World J. Microbiol. Biotechnol. 2011, 27, 2287–2295. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Q.; Wei, G.; Liang, Q.; Qi, Q. Production in Escherichia coli of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with differing monomer compositions from unrelated carbon sources. Appl. Environ. Microbiol. 2011, 77, 4886–4893. [Google Scholar] [CrossRef] [Green Version]
- Huijberts, G.N.; Eggink, G.; De Waard, P.; Huisman, G.W.; Witholt, B. Pseudomonas putida KT2442 cultivated on glucose accumulates poly(3-hydroxyalkanoates) consisting of saturated and unsaturated monomers. Appl. Environ. Microbiol. 1992, 58, 536–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majid, M.I.A.; Akmal, D.H.; Few, L.L.; Agustien, A.; Toh, M.S.; Samian, M.R.; Najimudin, N.; Azizan, M.N. Production of poly(3-hydroxybutyrate) and its copolymer poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Erwinia sp. USMI-20. Int. J. Biol. Macromol. 1999, 25, 95–104. [Google Scholar] [CrossRef]
- Bingham, E.; Cohrssen, B.; Powell, C.H. Patty’s Toxicology Volume 1–6, 6th ed.; John Wiley & Sons: New York, NY, USA, 2012. [Google Scholar]
- Klabunde, J.; Bischoff, C.; Papa, A.J. Propanols. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2018; pp. 1–14. Available online: https://onlinelibrary.wiley.com/doi/10.1002/14356007.a22_173.pub3 (accessed on 15 January 2022).
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on solid catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Lütke-Eversloh, T. Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochem. Eng. J. 2003, 16, 81–96. [Google Scholar] [CrossRef]
- Berezina, N.; Yada, B. Improvement of the poly(3-hydroxybutyrate-co-3-hydroxyvalerate)(PHBV) production by dual feeding with levulinic acid and sodium propionate in Cupriavidus necator. New Biotechnol. 2016, 33, 231–236. [Google Scholar] [CrossRef]
- Chung, S.H.; Park, G.G.; Kim, H.W.; Rhee, Y.H. Effect of levulinic acid on the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Ralstonia eutropha KHB-8862. J. Microbiol. 2001, 39, 79–82. [Google Scholar]
- Kim, D.Y.; Park, D.S.; Kwon, S.B.; Chung, M.G.; Bae, K.S.; Park, H.Y.; Rhee, Y.H. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolyesters with a high molar fraction of 3-hydroxyvalerate by an insect-symbiotic Burkholderia sp. IS-01. J. Microbiol. 2009, 47, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Hesse, P.; Fasl, H.; Stelzer, F.; Braunegg, G. Study on the effect of levulinic acid on whey-based biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Hydrogenophaga pseudoflava. Appl. Food Biotechnol. 2017, 4, 65–78. [Google Scholar] [CrossRef]
- Park, S.K.; Lee, K.T.; Kim, Y.B.; Rhee, Y.H. Biosynthesis of polyhydroxybutyrate and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Bacillus thuringiensis R-510. J. Microbiol. 1997, 35, 127–133. [Google Scholar]
- Gahlawat, G.; Soni, S.K. Valorization of waste glycerol for the production of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer by Cupriavidus necator and extraction in a sustainable manner. Bioresour. Technol. 2017, 243, 492–501. [Google Scholar] [CrossRef]
- Grousseau, E.; Blanchet, E.; Déléris, S.; Albuquerque, M.G.; Paul, E.; Uribelarrea, J.L. Phosphorus limitation strategy to increase propionic acid flux towards 3-hydroxyvaleric acid monomers in Cupriavidus necator. Bioresour. Technol. 2014, 153, 206–215. [Google Scholar] [CrossRef]
- Khanna, S.; Srivastava, A.K. Production of poly(3-hydroxybutyric-co-3-hydroxyvaleric acid) having a high hydroxyvalerate content with valeric acid feeding. J. Ind. Microbiol. Biotechnol. 2007, 34, 457–461. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, S.C.; Lee, S.Y.; Chang, H.N.; Chang, Y.K.; Woo, S.I. Production of poly(3-hydroxybutyric-co-3-hydroxyvaleric acid) by fed-batch culture of Alcaligenes eutrophus with substrate control using on-line glucose analyzer. Enzym. Microb. Technol. 1994, 16, 556–561. [Google Scholar] [CrossRef]
- Dionisi, D.; Majone, M.; Papa, V.; Beccari, M. Biodegradable polymers from organic acids by using activated sludge enriched by aerobic periodic feeding. Biotechnol. Bioeng. 2004, 85, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Saha, N.R.; Pal, A.; Chattopadhyay, D.; Paul, A.K. Comparative evaluation of physicochemical characteristics of biopolyesters P(3HB) and P(3HB-co-3HV) produced by endophytic Bacillus cereus RCL 02. Front. Biol. 2018, 13, 297–308. [Google Scholar] [CrossRef]
- Amirul, A.A.; Syairah, S.N.; Yahya, A.R.; Azizan, M.N.M.; Majid, M.I.A. Synthesis of biodegradable polyesters by Gram-negative bacterium isolated from Malaysian environment. World J. Microbiol. Biotechnol. 2008, 24, 1327–1332. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, P.; Kim, J.H. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from Methylobacterium organophilum by potassium-limited fed-batch culture. Enzym. Microb. Technol. 1999, 24, 555–560. [Google Scholar] [CrossRef]
- Sheu, D.S.; Chen, W.M.; Yang, J.Y.; Chang, R.C. Thermophilic bacterium Caldimonas taiwanensis produces poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from starch and valerate as carbon sources. Enzym. Microb. Technol. 2009, 44, 289–294. [Google Scholar] [CrossRef]
- Myung, J.; Galega, W.M.; Van Nostrand, J.D.; Yuan, T.; Zhou, J.; Criddle, C.S. Long-term cultivation of a stable Methylocystis-dominated methanotrophic enrichment enabling tailored production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Bioresour. Technol. 2015, 198, 811–818. [Google Scholar] [CrossRef]
- Amini, M.; Sobhani, S.; Younesi, H.; Abyar, H.; Salamatinia, B.; Mohammadi, M. Evaluating the feasibility of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) co-biopolymer production from rice wastewater by Azohydromonas lata. Appl. Food Biotechnol. 2020, 7, 73–83. [Google Scholar] [CrossRef]
- Matsumoto, K.I.; Kitagawa, K.; Jo, S.J.; Song, Y.; Taguchi, S. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in recombinant Corynebacterium glutamicum using propionate as a precursor. J. Biotechnol. 2011, 152, 144–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doi, Y.; Kunioka, M.; Nakamura, Y.; Soga, K. Biosynthesis of copolyesters in Alcaligenes eutrophus H16 from carbon-13 labeled acetate and propionate. Macromolecules 1987, 20, 2988–2991. [Google Scholar] [CrossRef]
- Bhubalan, K.; Lee, W.H.; Loo, C.Y.; Yamamoto, T.; Tsuge, T.; Doi, Y.; Sudesh, K. Controlled biosynthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) from mixtures of palm kernel oil and 3HV-precursors. Polym. Degrad. Stab. 2008, 93, 17–23. [Google Scholar] [CrossRef]
- Catalán, A.I.; Malan, A.K.; Ferreira, F.; Gill, P.R.; Batista, S. Propionic acid metabolism and poly-3-hydroxybutyrate-co-3-hydroxyvalerate production by a prpC mutant of Herbaspirillum seropedicae Z69. J. Biotechnol. 2018, 286, 36–44. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.Y.; Nomura, C.T.; Sudesh, K. Biosynthesis of polyhydroxyalkanoate copolymers from mixtures of plant oils and 3-hydroxyvalerate precursors. Bioresour. Technol. 2008, 99, 6844–6851. [Google Scholar] [CrossRef]
- Cal, A.J.; Sikkema, W.D.; Ponce, M.I.; Franqui-Villanueva, D.; Riiff, T.J.; Orts, W.J.; Pieja, A.J.; Lee, C.C. Methanotrophic production of polyhydroxybutyrate-co-hydroxyvalerate with high hydroxyvalerate content. Int. J. Biol. Macromol. 2016, 87, 302–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, H.S.J.; Huong, K.H.; Hani, S.N.A.; Amirul, A.A.A. Genetic incorporation of oil-utilizing ability in Cupriavidus malaysiensis USMAA2-4 for sustainable polyhydroxyalkanoates production from palm olein and 1-pentanol. J. Biotechnol. 2021, 337, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Huong, K.H.; Shantini, K.; Sharmini, R.; Amirul, A.A. Exploring the potential of 1-pentanol and oleic acid for optimizing the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer by Cupriavidus sp. USMAA1020. Arab. J. Sci. Eng. 2017, 42, 2313–2320. [Google Scholar] [CrossRef]
- Kiun, J.T.; Amelia, T.S.M.; Huong, K.H.; Amirul, A.A.; Bhubalan, K. Optimizing the biosynthesis of renewable polyhydroxyalkanoate copolymer containing 3-hydroxyvalerate by Massilia haematophila using statistical modeling. BioTechnologia 2019, 100, 359–371. [Google Scholar] [CrossRef]
- Ezhov, V.A.; Doronina, N.V.; Trotsenko, Y.A. Biosynthesis of polyhydroxybutyrate/valerate with different molecular weights during the growth of Methylobacterium extorquens G-10 on a methanol-pentanol mixture. Appl. Biochem. Microbiol. 2013, 49, 150–153. [Google Scholar] [CrossRef]
- Galuzina, T.V.; Gerasin, V.A.; Doronina, N.V.; Ezhov, V.A.; Trotsenko, Y.A.; Kiprianov, S.V.; Ivanov, A.O.; Filatova, M.P.; Shklyaruk, B.F. Structures and properties of polyhydroxyalkanoates synthesized by Methyloligella halotolerans C2 and Methylobacterium extorquens G10 from a methanol–pentanol mixture. Polym. Sci. Ser. A 2015, 57, 729–737. [Google Scholar] [CrossRef]
- Raberg, M.; Voigt, B.; Hecker, M.; Steinbüchel, A. A closer look on the polyhydroxybutyrate-(PHB)-negative phenotype of Ralstonia eutropha PHB-4. PLoS ONE 2014, 9, e95907. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Lee, S.Y. Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl. Microbiol. Biotechnol. 1999, 51, 13–21. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.K.; Saratale, G.D.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Bharagava, R.N.; Kumar, G.; Kim, D.S.; Mulla, S.I.; et al. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685–124699. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Jana, K.; Haldar, S.; Bhowmic, A.; Mukhopadhyay, U.K.; De, S.; Mukherjee, J. Integration of poly-3-(hydroxybutyrate-co-hydroxyvalerate) production by Haloferax mediterranei through utilization of stillage from rice-based ethanol manufacture in India and its techno-economic analysis. World J. Microbiol. Biotechnol. 2015, 31, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, A.M. 6.02–Hydroformylation. In Comprehensive Inorganic Chemistry II, 2nd ed.; Reedijk, J., Poeppelmeier, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 25–46. [Google Scholar] [CrossRef]
- Torres, G.M.; Frauenlob, R.; Franke, R.; Börner, A. Production of alcohols via hydroformylation. Catal. Sci. Technol. 2015, 5, 34–54. [Google Scholar] [CrossRef]
- Molnár, Á.; Papp, A. Catalyst recycling—a survey of recent progress and current status. Coord. Chem. Rev. 2017, 349, 1–65. [Google Scholar] [CrossRef]
- Lappe, P.; Hofmann, T. Pentanols. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2012; pp. 245–258. Available online: https://onlinelibrary.wiley.com/doi/10.1002/14356007.a19_049.pub2 (accessed on 15 January 2022).
- Cropley, J.B.; Burgess, L.M.; Loke, R.A. Butyraldehyde hydrogenation-a case-study in process design. Chemtech 1984, 14, 374–380. [Google Scholar]
- Faith, W.L.; Keyes, D.B.; Clark, R.L. Industrial Chemicals, 3rd ed.; Wiley & Sons: New York, NY, USA, 1965. [Google Scholar]
- Fuchs, D.; Rousseau, G.; Diab, L.; Gellrich, U.; Breit, B. Tandem rhodium-catalyzed hydroformylation–hydrogenation of alkenes by employing a cooperative ligand system. Angew. Chem. Int. Ed. 2012, 51, 2178–2182. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.H.G.; Kellermeyer, R.W.; Stjernholm, R.L.; Wood, H.G. Purification and properties of enzymes involved in the propionic acid fermentation. J. Bacteriol. 1964, 87, 171–187. [Google Scholar] [CrossRef] [Green Version]
- Wood, H.G. Metabolic cycles in the fermentation by propionic acid bacteria. In Current Topics in Cellular Regulation; Academic Press: Cambridge, MA, USA, 1981; Volume 18, pp. 255–287. [Google Scholar] [CrossRef]
- Himmi, E.H.; Bories, A.; Boussaid, A.; Hassani, L. Propionic acid fermentation of glycerol and glucose by Propionibacterium acidipropionici and Propionibacterium freudenreichii ssp. shermanii. Appl. Microbiol. Biotechnol. 2000, 53, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, S.; Janssen, P.H.; Schink, B. Energetics and kinetics of lactate fermentation to acetate and propionate via methylmalonyl-CoA or acrylyl-CoA. FEMS Microbiol. Lett. 2002, 211, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.; François, J.M. Microbial production of propanol. Biotechnol. Adv. 2016, 34, 984–996. [Google Scholar] [CrossRef]
- Cardon, B.P.; Barker, H.A. Two new amino-acid-fermenting bacteria, Clostridium propionicum and Diplococcus glycinophilus. J. Bacterial. 1946, 52, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Tholozan, J.L.; Touzel, J.P.; Samain, E.; Grivet, J.P.; Prensier, G.; Albagnac, G. Clostridium neopropionicum sp. nov., a strict anaerobic bacterium fermenting ethanol to propionate through acrylate pathway. Arch. Microbiol. 1992, 157, 249–257. [Google Scholar] [CrossRef]
- Liu, K.; Atiyeh, H.K.; Stevenson, B.S.; Tanner, R.S.; Wilkins, M.R.; Huhnke, R.L. Continuous syngas fermentation for the production of ethanol, n-propanol and n-butanol. Bioresour. Technol. 2014, 151, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Millat, T.; Janssen, H.; Thorn, G.J.; King, J.R.; Bahl, H.; Fischer, R.J.; Wolkenhauer, O. A shift in the dominant phenotype governs the pH-induced metabolic switch of Clostridium acetobutylicum in phosphate-limited continuous cultures. Appl. Microbiol. Biotechnol. 2013, 97, 6451–6466. [Google Scholar] [CrossRef] [PubMed]
- Isom, C.E.; Nanny, M.A.; Tanner, R.S. Improved conversion efficiencies for n-fatty acid reduction to primary alcohols by the solventogenic acetogen “Clostridium ragsdalei”. J. Ind. Microbiol. Biotechnol. 2015, 42, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, S.; Liao, J.C. Directed evolution of Methanococcus jannaschii citramalate synthase for biosynthesis of 1-propanol and 1-butanol by Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 7802–7808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.R.; Liao, J.C. Metabolic engineering of Escherichia coli for 1-butanol and 1-propanol production via the keto-acid pathways. Metab. Eng. 2008, 10, 312–320. [Google Scholar] [CrossRef]
- Shen, C.R.; Liao, J.C. Synergy as design principle for metabolic engineering of 1-propanol production in Escherichia coli. Metab. Eng. 2013, 17, 12–22. [Google Scholar] [CrossRef]
- Chen, G.S.; Siao, S.W.; Shen, C.R. Saturated mutagenesis of ketoisovalerate decarboxylase V461 enabled specific synthesis of 1-pentanol via the ketoacid elongation cycle. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Haller, T.; Buckel, T.; Rétey, J.; Gerlt, J.A. Discovering new enzymes and metabolic pathways: Conversion of succinate to propionate by Escherichia coli. Biochemistry 2000, 39, 4622–4629. [Google Scholar] [CrossRef]
- Srirangan, K.; Liu, X.; Westbrook, A.; Akawi, L.; Pyne, M.E.; Moo-Young, M.; Chou, C.P. Biochemical, genetic, and metabolic engineering strategies to enhance coproduction of 1-propanol and ethanol in engineered Escherichia coli. Appl. Microbiol. Biotechnol. 2015, 98, 9499–9515. [Google Scholar] [CrossRef]
- Park, H.; Jeon, B.S.; Sang, B.I. efficient, simple production of corresponding alcohols from supplemented C2-C8 carboxylic acids in Escherichia coli using acyl-CoA transferase from Megasphaera hexanoica. Biotechnol. Bioprocess. Eng. 2020, 25, 599–606. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Schlegel, H.G. Physiology and molecular genetics of poly(β-hydroxyalkanoic acid) synthesis in Alcaligenes eutrophus. Mol. Microbiol. 1991, 5, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.P.; Rainey, F.A.; Wood, A.P. The genus Paracoccus. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 232–249. [Google Scholar] [CrossRef]
- Akmal, D.; Asiska, P.D.; Wangi, Q.A.; Rivai, H.; Agustien, A. Biosynthesis of copolymer poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from palm oil and n-pentanol in a 10 L bioreactor. Rasayan J. Chem. 2015, 8, 389–395. [Google Scholar]
- Amirul, A.A.; Yahya, A.R.; Sudesh, K.; Azizan, M.N.M.; Majid, M.I.A. Isolation of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) producer from Malaysian environment using γ-butyrolactone as carbon source. World J. Microbiol. Biotechnol. 2009, 25, 1199–1206. [Google Scholar] [CrossRef]
- Ramachandran, H.; Shafie, N.A.H.; Sudesh, K.; Azizan, M.N.; Majid, M.I.A.; Amirul, A.A.A. Cupriavidus malaysiensis sp. nov.; a novel poly(3-hydroxybutyrate-co-4-hydroxybutyrate) accumulating bacterium isolated from the Malaysian environment. Antonie Leeuwenhoek 2018, 111, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Shantini, K.; Bhubalan, K.; Yahya, A.R.M.; Amirul, A.A. Productivity increment of biodegradable and biorenewable copolymer containing 3-hydroxyvalerate monomer initiated by alcohols as precursor substrates. J. Chem. Technol. Biotechnol. 2013, 88, 1364–1370. [Google Scholar] [CrossRef]
- Sashi, P.; Yasin, U.M.; Bhuyan, A.K. Unfolding action of alcohols on a highly negatively charged state of cytochrome c. Biochemistry 2012, 51, 3273–3283. [Google Scholar] [CrossRef]
- Ingram, L.O. Adaptation of membrane lipids to alcohols. J. Bacterial. 1976, 125, 670–678. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, G.; Lloyd, L.J.; Stumpf, M.P.; Mayhew, A.J.; Buck, M. Induction and function of the phage shock protein extracytoplasmic stress response in Escherichia coli. J. Biol. Chem. 2006, 281, 21147–21161. [Google Scholar] [CrossRef] [Green Version]
- Yano, T.; Miyahara, Y.; Morii, N.; Okano, T.; Kubota, H. Pentanol and benzyl alcohol attack bacterial surface structures differently. Appl. Environ. Microbiol. 2016, 82, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Osman, Y.A.; Ingram, L.O. Mechanism of ethanol inhibition of fermentation in Zymomonas mobilis CP4. J. Bacterial. 1985, 164, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Ingram, L.O.N.; Buttke, T.M. Effects of alcohols on micro-organisms. Adv. Microb. Physiol. 1985, 25, 253–300. [Google Scholar] [CrossRef]
- Heipieper, H.J.; Meinhardt, F.; Segura, A. The cis–trans isomerase of unsaturated fatty acids in Pseudomonas and Vibrio: Biochemistry, molecular biology and physiological function of a unique stress adaptive mechanism. FEMS Microbiol. Lett. 2003, 229, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Beaven, M.J.; Charpentier, C.; Rose, A.H. Production and tolerance of ethanol in relation to phospholipid fatty-acyl composition in Saccharomyces cerevisiae NCYC 431. Microbiology 1982, 128, 1447–1455. [Google Scholar] [CrossRef] [Green Version]
- Kitahara, K.; Takichi, K.; Osamu, G. Taxonomic studies on the hiochi-bacteria, specific saprophytes of sake II. Identification and classification of hiochi-bacteria. J. Gen. Appl. Microbiol. 1957, 3, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Bernal, P.; Muñoz-Rojas, J.; Hurtado, A.; Ramos, J.L.; Segura, A. A Pseudomonas putida cardiolipin synthesis mutant exhibits increased sensitivity to drugs related to transport functionality. Environ. Microbiol. 2007, 9, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Marr, A.G.; Ingraham, J.L. Effect of temperature on the composition of fatty acids in Escherichia coli. J. Bacteriol. 1962, 84, 1260–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brynildsen, M.P.; Liao, J.C. An integrated network approach identifies the isobutanol response network of Escherichia coli. Mol. Syst. Biol. 2009, 5, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.L.; Dassa, E.; Orelle, C.; Chen, J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 2008, 72, 317–364. [Google Scholar] [CrossRef] [Green Version]
- Rutherford, B.J.; Dahl, R.H.; Price, R.E.; Szmidt, H.L.; Benke, P.I.; Mukhopadhyay, A.; Keasling, J.D. Functional genomic study of exogenous n-butanol stress in Escherichia coli. Appl. Environ. Microbiol. 2010, 76, 1935–1945. [Google Scholar] [CrossRef] [Green Version]
- Hews, C.L.; Cho, T.; Rowley, G.; Raivio, T.L. Maintaining integrity under stress: Envelope stress response regulation of pathogenesis in Gram-negative bacteria. Front. Cell. Infect. Microbiol. 2019, 9, 313–337. [Google Scholar] [CrossRef] [Green Version]
- Isaac, D.D.; Pinkner, J.S.; Hultgren, S.J.; Silhavy, T.J. The extracytoplasmic adaptor protein CpxP is degraded with substrate by DegP. Proc. Natl. Acad. Sci. USA 2005, 102, 17775–17779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, R.; Gadkari, V.V.; Meinen, B.A.; van Mierlo, C.P.; Ruotolo, B.T.; Bardwell, J.C. Mechanism of the small ATP-independent chaperone Spy is substrate specific. Nat. Commun. 2021, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tomas, C.A.; Welker, N.E.; Papoutsakis, E.T. Overexpression of groESL in Clostridium acetobutylicum results in increased solvent production and tolerance, prolonged metabolism, and changes in the cell’s transcriptional program. Appl. Environ. Microbiol. 2003, 69, 4951–4965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postma, P.W.; Lengeler, J.W.; Jacobson, G.R. Phosphoenolpyruvate: Carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993, 57, 543–594. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Fujita, N.; Ishihama, A. Sequence analysis of two temperature-sensitive mutations in the alpha subunit gene (rpoA) of Escherichia coli RNA polymerase. Nucleic Acids Res. 1990, 18, 5945–5948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafri, S.; Urbanowski, M.L.; Stauffer, G.V. A mutation in the rpoA gene encoding the alpha subunit of RNA polymerase that affects metE-metR transcription in Escherichia coli. J. Bacteriol. 1995, 177, 524–529. [Google Scholar] [CrossRef] [Green Version]
- Klein-Marcuschamer, D.; Santos, C.N.S.; Yu, H.; Stephanopoulos, G. Mutagenesis of the bacterial RNA polymerase alpha subunit for improvement of complex phenotypes. Appl. Environ. Microbiol. 2009, 75, 2705–2711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peck, M.C.; Gaal, T.; Fisher, R.F.; Gourse, R.L.; Long, S.R. The RNA polymerase α subunit from Sinorhizobium meliloti can assemble with RNA polymerase subunits from Escherichia coli and function in basal and activated transcription both in vivo and in vitro. J. Bacteriol. 2002, 184, 3808–3814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, M.S.; Glass, R.E. Escherichia coli rpoA mutation which impairs transcription of positively regulated systems. Mol. Microbiol. 1991, 5, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Kidwell, J.; Valentin, H.E.; Dennis, D. Regulated expression of the Alcaligenes eutrophus pha biosynthesis genes in Escherichia coli. Appl. Environ. Microbiol. 1995, 61, 1391–1398. [Google Scholar] [CrossRef] [Green Version]
- Nomura, C.T.; Taguchi, K.; Taguchi, S.; Doi, Y. Coexpression of genetically engineered 3-ketoacyl-ACP synthase III (fabH) and polyhydroxyalkanoate synthase (phaC) genes leads to short-chain-length-medium-chain-length polyhydroxyalkanoate copolymer production from glucose in Escherichia coli JM109. Appl. Environ. Microbiol. 2004, 70, 999–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taguchi, K.; Aoyagi, Y.; Matsusaki, H.; Fukui, T.; Doi, Y. Over-expression of 3-ketoacyl-ACP synthase III or malonyl-CoA-ACP transacylase gene induces monomer supply for polyhydroxybutyrate production in Escherichia coli HB101. Biotechnol. Lett. 1999, 21, 579–584. [Google Scholar] [CrossRef]











| Incorporated Components A | Changes in the Properties | Potential Applications | Ref. |
|---|---|---|---|
| α-P(3HB) Incorporation method: Solvent casting 3HV fraction: 10 mol% | P(3HB-co-3HV):α-P(3HB) (100:0 → 50:50) Melting temperature: 145 → 133 °C Degree of crystallinity: 61% → 30% Tensile strength: 27 → 7 MPa Elongation at break: 1% → 29% Young’s modulus: 1500 → 240 MPa Enzymatic degradation: 85% → 94% | Packaging material | [25] |
| AS Incorporation method: Solvent casting 3HV fraction: 59 mol% | P(3HB-co-3HV)/P(3HB-co-3HV):AS Melting temperature: 275.84 °C/294.97 °C Degree of crystallinity: 98.96%/98.23% Free radical scavenging activity (24 h): 1%/14% Incubation biodegradation (day 6): smooth surface/small pits | Therapeutic implant | [26] |
| CNC Incorporation method: Solvent casting 3HV fraction: 12 mol% | P(3HB-co-3HV):CNC (100:0 → 94:6) Melting temperature: 136.8 → 151.1 °C Crystallization temperature: 96.5 → 101.2 °C Degree of crystallinity: 49.9% → 57.5% Water vapor transmission rate: 308 → 115 g m−2 day−1 Oxygen transfer rate: 425 → 113 cm m−2 day−1 | Packaging material | [27] |
| DDGS or Misc Incorporation method: Twin screw extrusion 3HV fraction: 5 mol% | P(3HB-co-3HV):DDGS (100:0/85:15/75:25) Tensile strength: 8.5 MPa/6.0 MPa/4.8 MPa Young’s modulus: 3.9 GPa/3.9 GPa/3.8 GPa Flexural strength: 7.0 MPa/5.8 MPa/4.7 MPa Flexural modulus: 4.8 GPa/4.6 GPa/4.4 GPa CO2 evolution (day 320): 155 mg/175 mg/200 mg Marine biodegradation (day 320): 73%/90%/100% P(3HB-co-3HV):Misc (85:15/75:25) Tensile strength: 8.8 MPa/8.9 MPa Young’s modulus: 5.9 GPa/7.7 GPa Flexural strength: 7.8 MPa/7.4 MPa Flexural modulus:5.6 GPa/6.6 GPa CO2 evolution (day 320):175 mg/180 mg Marine biodegradation (day 320): 84%/88% | Packaging material | [28] |
| Eugenol Incorporation method: Electrospinning 3HV fraction: 3 mol% | P(3HB-co-3HV):Eugenol (100:0 → 85:15) Temperature of 5% weight loss: 276.6 → 160.8 °C Degradation temperature: 304.7 → 293.3 °C Mass loss at degradation temperature: 61.01% → 76.36% Water vapor permeability: 4.05 × 1014→ 0.95 × 1014 Kg m m−2 s−1 Pa−1 Limonene vapor permeability: 3.75 → 0.81 Kg m m−2 s−1 Pa−1 Water vapor permeance: 5.87 → 1.33 Kg m m−2 s−1 Pa−1 Limonene vapor permeance: 5.44 → 1.14 Kg m m−2 s−1 Pa−1 Tensile strength: 1252 → 1897 MPa Elongation at break: 2.0% → 2.5% Young’s modulus: 18.1 → 26.5 MPa S. aureus growth: 5.16 → 3.45 log(CFU mL−1) Escherichia coli growth: 5.79 → 3.88 log(CFU mL−1) | Antimicrobial food packaging | [29] |
| HA Incorporation method: Melt-pressing 3HV fraction: 8–24 mol% | P(3HB-co-3HV), 0→24 mol% 3HV Melting temperature: 170 → 129 °C Degree of crystallinity: 69% → 55% P(3HB-co-3HV):HA (30:70), 0 → 24 mol% 3HV Tensile strength: 67 → 23 MPa Elongation at break: 2.65% → 3.84% Young’s modulus: 2.52 → 0.47 GPa | Bone implant | [30] |
| MAT Incorporation method: Solvent casting 3HV fraction: 4 mol% | P(3HB-co-3HV):MAT (100:0 → 95:5) Melting temperature: 168.58 → 130.91 °C Glass transition temperature: −2.03 → −6.61 °C Crystallization temperature: 46.15 → 46.98 °C Degree of crystallinity: 53.7% → 36.8% | Packaging material | [31] |
| MCPA Incorporation method: Melt-blending and hot-pressing 3HV fraction: 3 mol% | P(3HB-co-3HV)-MCPA (95:5/90:10/85:15) Melting temperature 1: 123.2 °C/124.1 °C /NA Melting temperature 2: 150.7 °C/150.7 °C/140.9 °C Enthalpy of fusion 1:1944 J g−1/2482 J g−1/NA Enthalpy of fusion 2:1745 J g−1/1745 J g−1/1509 J g−1 Glass transition temperature 1: −28.2 °C/−28.0 °C/−27.4 °C Glass transition temperature 2: 48.6 °C/47.9 °C/36.9 °C Crystallization temperature: 102.4 °C/102.2 °C/99.0 °C Chlorine loss: 0.3%/1.3%/1.7% MCPA loss: 5.1%/7.4%/9.7% P(3HB-co-3HV) loss before bond scission: 20.6%/29.7%/38.8% P(3HB-co-3HV) loss after bond scission: 2.8%/2.4%/2.4% | Mulch | [32] |
| mPEG Incorporation method: Transesterification 3HV fraction: 12 and 33 mol% | P(3HB-co-3HV):mPEG, 12 mol%/33 mol% 3HV Number average molecular weight: 8980/4980 Weight average molecular weight: 6200/2650 Polydispersity index: 1.44/1.84 Melting temperature of P(3HB-co-3HV) block: 140.5 °C/133.6 °C Melting temperature of mPEG block: 49.1 °C/49.3 °C Particle size: 162 nm/125 nm Encapsulation efficiency: 43%/57% Cytotoxicity (100 → 500 µg/mL nanoparticles): 94% → 80%/88% → 78% | Drug delivery carrier | [24] |
| NH2-g-collagen or PHEMA-g-collagen Incorporation method: Solvent casting followed by solute leaching technique 3HV fraction: 12 mol% | Porous P(3HB-co-3HV) Decomposition temperature at 10% weight loss: 263.15 °C Collagen concentration: NA Ag/BSA load: 0.037 µg cm−2 Surface roughness: 0.1983 µm P(3HB-co-3HV)--g-PHEMA-g-collagen Decomposition temperature at 10% weight loss: 264.60 °C Collagen concentration: 29.93 µg cm−2 Ag/BSA load: 0.29 µg cm−2 Surface roughness: NA P(3HB-co-3HV)-g-NH2-g-collagen Decomposition temperature at 10% weight loss: 256.15 °C Collagen concentration: 55.16 µg cm−2 Ag/BSA load: 0.26 µg cm−2 Surface roughness: 0.2643 µm | Bone implant | [33] |
| NR Incorporation method: Twin screw extrusion 3HV fraction: 3 mol% | P(3HB-co-3HV):NR (100:0/85:15) Melting temperature: 172.05 °C/171.95 °C P(3HB-co-3HV) glass transition temperature: 5.65 °C/6.05 °C NR glass transition temperature: NA/−64.5 °C Crystallization temperature: 120.85 °C/119.45 °C Degree of crystallinity: 74.7%/61.6% Tensile strength: 43 MPa/26 MPa Elongation at break: 8%/16% Notched impact strength: 15 J m−1/14 J m−1 Secant modulus: 12 GPa/0.9 GPa | Packaging material | [34] |
| PBAT Incorporation method: Conventional injection molding or microcellular injection molding 3HV fraction: NA | Solid P(3HB-co-3HV):PBAT (98.5:1.5 → 30:70) Melting temperature: 166.2 → 170.4 °C Cold crystallization temperature: NA → 44.7 °C Degree of crystallinity: 78% → 29% Specific toughness: 5.3 x 10−4 → 7.1 x 10−2 MPa kg−1 m−3 Elongation at break: 2.7% → 555.7% Specific tensile strength: 3.2 × 10−2 → 1.5 x 10−2 MPa kg−1 m−3 Specific Young’s modulus: 2.2 → 0.5 MPa kg−1 m−3 Microcellular P(3HB-co-3HV):PBAT (98.5:1.5 → 30:70) Melting temperature: 167.1 → 169.6 °C Cold crystallization temperature: NA → 45.7 °C Degree of crystallinity: 80% → 25% Specific toughness: 3.8 x 10−4 → 5.8 x 10−2 MPa kg−1 m−3 Elongation at break: 2.2% → 493.9% Specific tensile strength: 2.7 × 10−2 → 1.3 x 10−2 MPa kg−1 m−3 Specific Young’s modulus: 2.1 → 0.5 MPa kg−1 m−3 | Packaging material | [35] |
| PBS Incorporation method: Solvent casting 3HV fraction: 14 mol% | P(3HB-co-3HV):PBS (100:0 → 40:60) Crystallization time at 60 °C: 8 → 14.5 min Overall crystallization constant: 3.13 × 10−2 → 2.22 × 10−3 min−n Avrami index: 2.57 → 2.67 | Packaging material | [36] |
| PBS-DCP Incorporation method: Compression molding 3HV fraction: 13 mol% | P(3HB-co-3HV):PBS (100:0 → 70:30) Tensile strength: 22 → 23 MPa Elongation at break: 4.5% → 6.5% 80 wt%P(3HB-co-3HV)–20 wt%PBS:DCP (100:0 → 99:1) Tensile strength: 25 → 27 MPa Elongation at break: 8% → 350% Notched Izod impact toughness: 2.8 → 5.5 kJ m−2 Flexural strength: 39 → 30 MPa Flexural modulus: 1.2 → 0.6 GPa | Packaging material | [37] |
| PCL Incorporation method: Solvent casting 3HV fraction: 7 mol% | P(3HB-co-3HV)/PCL Number average molecular weight: 127,000/56,400 Weight average molecular weight: 470,000/163,300 Melting temperature: 151.2 °C/64.0 °C Glass transition temperature: 5.2 °C/−61.0 °C Crystallization temperature: 97.0 °C/22.2 °C P(3HB-co-3HV):PCL (100:0 → 50:50) Isothermal crystallization temperature: 120 → 120 °C Overall crystallization constant: 2.20 × 10−7 → 1.00 × 10−8 s−n Avrami index: 2.80 → 2.66 | Packaging material | [38] |
| PDLLA-PEG Incorporation method: Compression molding 3HV fraction: 1 mol% | P(3HB-co-3HV):PDLLA (100:0 → 30:70) Melting temperature: 157.8 → 169.8 °C Degree of crystallinity: 53.6 → 9.9 °C Tensile strength: 19.7 → 49.7 MPa Elongation at break: 0.17% → 2.07% Flexural strength: 39.1 → 75.0 MPa Flexural modulus:3646 → 3507 MPa Burial biodegradation (day 30): 0% → 1% 30 wt%P(3HB-co-3HV)–70 wt%PDLLA:PEG (90:10 → 80:20) Melting temperature: 171.2 → 170.8 °C Degree of crystallinity: 10.5 → 13.0 °C Tensile strength: 29.7 → 24.1 MPa Elongation at break: 28.7% → 237.0% Flexural strength: 36.1 → 5.48 MPa Flexural modulus: 1127 → 220 MPa Burial biodegradation (day 30): 3% → 11% | Biomedical, agricultural and packaging material | [39] |
| PEG Incorporation method: Solvent casting 3HV fraction: 4 mol% | P(3HB-co-3HV):PEG (100:0 → 20:80) Melting temperature: 163.2 → 145.0 °C Enthalpy of fusion: 89.62 → 1.63 J g−1 | Drug delivery carrier | [22] |
| PEG Incorporation method: Solvent casting 3HV fraction: NA | P(3HB-co-3HV) Melting temperature: 90 °C Initial thermal degradation temperature: 220 °C Final thermal degradation temperature: 255 °C Tensile strength: 10.3 MPa Elongation at break: 13.3% Cytotoxicity: 20% P(3HB-co-3HV):PEG (4:1) Cytotoxicity: 0%–10% | Skin grafting | [40] |
| PLA-CNT Incorporation method: High-speed spinning 3HV fraction: 2 mol% | P(3HB-co-3HV)/PLA Melting temperature: 172 °C/170 °C Glass transition temperature: 5 °C/64 °C Enthalpy of fusion: 92.8 J g−1/44.2 J g−1 Crystallization temperature: 122 °C/112 °C Decomposition temperature: 303 °C/382 °C Izod impact strength: 1.99 kJ m−2/2.14 kJ m−2 Flexural strength: 47.70 MPa/58.07 MPa Flexural modulus: 3.48 GPa/2.94 GPa 80 wt%P(3HB-co-3HV)–20 wt%PLA:CNT (100:0 → 99:1) Melting temperature: 169 → 168 °C Glass transition temperature: −2 → −2 °C Enthalpy of fusion: 44.11 → 48.10 J g−1 Crystallization temperature: 112 → 122 °C Decomposition temperature: 379 → 380 °C Izod impact strength: 4.10 → 2.46 kJ m−2 Flexural strength: 51.60 → 61.01 MPa Flexural modulus: 3.10 → 3.25 GPa Electrical conductivity: 8.67 × 10−14 → 2.79 × 10−2 S m−1 Reflectivity (frequency): 0 dB (NA) → −15 dB (11 GHz) | Electrical and electromagnetic | [41] |
| PLA-nanoclay Incorporation method: Twin screw extrusion 3HV fraction: NA | P(3HB-co-3HV):PLA (15:85 → 30:70) Melting temperature: 154.75 → 156.40 °C Cold crystallization temperature: 133.45 → 121.89 °C Degree of crystallinity: 1.98% → 4.33% Tensile strength: 52.5 → 47.5 MPa Elongation at break: 9.0% → 6.0% Young’s modulus: 1700 → 1750 MPa P(3HB-co-3HV)-PLA:nanoclay (15:85 → 30:70) Melting temperature: 156.52 → 157.43 °C Cold crystallization temperature: 129.09 → 111.04 °C Degree of crystallinity: 13.05% → 18.40% Tensile strength: 49.2 → 48.0 MPa Elongation at break: 8.5% → 4.0% Young’s modulus: 2060 → 2060 MPa | Packaging material | [42] |
| PPC Incorporation method: Solvent casting 3HV fraction: 5 mol% | P(3HB-co-3HV):PPC (100:0 → 20:80) Melting temperature: 163 → 162 °C Thermal decomposition temperature: 199 → 190 °C Maximum mass loss rate temperature: 286 → 267 °C Burial biodegradation: 100% (day 12) → 85% (day 30) | Packaging material | [43] |
| starch, cellulose or alginate Incorporation method: Solvent casting 3HV fraction: 6 mol% | P(3HB-co-3HV)-starch (100:0 → 30:70) Tensile strength: 25 → 1 MPa Elongation at break: 8% → 4% Young’s modulus: 181 → 4 MPa Density: 0.974 → 1.243 g cm−3 Solubility: 0% → 6.0% Water absorption capacity: 0% → 21.0% Burial biodegradation (day 30): 10% → 100% Immersion biodegradation (day 30): 23% → 100% P(3HB-co-3HV)-cellulose (100:0 → 30:70) Tensile strength: 25 → 1 MPa Elongation at break: 8% → 3% Young’s modulus: 181 → 7 MPa Density: 0.974 → 1.212 g cm−3 Solubility: 0% → 1.7% Water absorption capacity: 0% → 4.7% Burial biodegradation (day 30): 10% → 70% Immersion biodegradation (day 30): 23% → 100% P(3HB-co-3HV)-arginate (100:0 → 30:70) Tensile strength: 25 → 1 MPa Elongation at break: 8% → 2% Young’s modulus: 181 → 3 MPa Density: 0.974 → 1.053 g cm−3 Solubility: 0% → 19.0% Water absorption capacity: 0% → 33.0% Burial biodegradation (day 30): 10% → 80% Immersion biodegradation (day 30): 21% → 100% | Mulch | [44] |
| ZnO Incorporation method: Melt-mixed compression molding, electrospinning or coating 3HV fraction: 3 and 18 mol% | P(3HB-co-3 mol%3HV)/ P(3HB-co-18 mol%3HV) Melting temperature: 168.7 °C/170.9 °C Decomposition temperature: 290.8 °C/283.1 °C Crystallization temperature: 114.7 °C/101.0 °C Degree of crystallinity: 66%/63% Tensile strength: 33.9 MPa/18.5 MPa Elongation at break: 1.5%/1.3% Young’s modulus: 2.6 GPa/2.2 GPa L*, a*, b*: 82.3, 1.4, 17.7/32.7, 6.7, 10.2 PHBVs-D/PHBVs-P/PHBVs-C B Melting temperature: 166.9 °C/166.5 °C/169.0 °C Decomposition temperature: 271.3 °C/270.3 °C/270.8 °C Crystallization temperature: 112.1 °C/111.6 °C/118.0 °C Degree of crystallinity: 50%/51%/35% Tensile strength: 12.5 MPa/34.8 MPa/22.6 MPa Elongation at break: 6.5%/2.3%/6.2% Young’s modulus: 1.5 GPa/2.1 GPa/1.4 GPa L*, a*, b*: 56.9, 9.0, 25.3/58.4, 8.5, 25.1/72.5, 3.8, 24.7 | Active food packaging and food contact surface applications | [45] |
| ZnO Incorporation method: Laser 3D molding 3HV fraction: NA | P(3HB-co-3HV)-ZnO (100:0→95:5) Melting temperature: 171 → 158 °C Decomposition temperature: 261.2 → 288.7 °C Strain:14.0% → 9.5% Stress: 3.5 → 4.5 MPa Compression strength: 4 → 5 MPa Compression modulus: 60 → 80 MPa Bacterial inhibition rate (day 5): 2.5% → 79.0% Zn2+ release in deionized water (day 7): 0.19 → 0.34 mg L−1 | Bone repair | [46] |
| Microorganisms and Carbon Sources | Biomass (g/L) | PHA Content | 3HV Composition | 3HV Yield (g/g) | Ref. | ||
|---|---|---|---|---|---|---|---|
| (wt%) | (g/L) | (mol%) | (g/L) | ||||
| Organic acids | |||||||
| Bacillus aryabhattai PHB10 Glucose (20.0 g/L) Propionic acid (0.7 g/L) | 3.9 | 72 | 2.8 | - | - | - | [28] |
| Bacillus thuringiensis R-510 Glucose (23.5 g/L) Propionic acid (1.0 g/L) | 2.9 | 21 | 0.6 | 41 | 0.2 | 0.25 | [69] |
| C. necator DSM 545 Waste glycerol (20.0 g/L) Propionic acid (4.0 g/L) | 4.5 | 57 | 2.6 | 25 | 0.7 | 0.16 | [70] |
| C. necator DSM 545 Butyric acid (246.0 g/L) Propionic acid (186.0 g/L) | 65.9 | 88 | 58 | 36 | 20.8 | 0.11 | [71] |
| C. necator NRRL B 14690 Fructose (40.0 g/L) Propionic acid (4.0 g/L) | 8.2 | 73 | 6.0 | 23 | 1.4 | 0.35 | [72] |
| C. necator NCIMB 11599 Glucose (maintained at 10.0–20.0 g/L) Propionic acid (0.52 mol/mol glucose) | 112.3 | 57 | 64.0 | 14 | 15.7 | - | [73] |
| Erwinia sp. USMI-20 Palm oil (4.6 g/L) Propionic acid (1.9 g/L) | 4.2 | 40 | 1.7 | 34 | 0.6 | 0.30 | [60] |
| Activated sludge mixed culture Acetic acid, lactic acid, propionic acid | - | - | - | 31–66 | - | - | [74] |
| Bacillus cereus RCL 02 Glucose (25.0 g/L) Valeric acid (1.9 g/L) | 8.1 | 72 | 5.8 | 15 | 0.9 | 0.46 | [75] |
| C. malaysiensis USMAA9-39 Oleic acid (6.5 g/L) Valeric acid (0.9 g/L) | 5.2 | 43 | 2.2 | 17 | 0.4 | 0.42 | [76] |
| C. necator DSM 545 Waste glycerol (20.0 g/L) Valeric acid (4.0 g/L) | 5.3 | 64 | 3.4 | 31 | 1.1 | 0.26 | [70] |
| C. necator NRRL B 14690 Fructose (40.0 g/L) Valeric acid (4.0 g/L) | 7.2 | 40 | 2.9 | 62 | 1.8 | 0.45 | [72] |
| Erwinia sp. USMI-20 Palm oil (4.6 g/L) Valeric acid (2.0 g/L) | 4.8 | 34 | 1.6 | 47 | 0.3 | 0.14 | [60] |
| Methylobacterium organophilum NCIB 11278 Methanol (4.0 g/L) Valeric aid (0.5 g/L) | 2.5 | 50 | 1.3 | 10 | 0.1 | 0.25 | [77] |
| Burkholderis sp. IS-01 Gluconate (20.0 g/L) Levulinic acid (12.5 g/L) | 5.9 | 62 | 3.7 | 87 | 3.2 | 0.25 | [67] |
| C. necator KHB-8862 Fructose syrup (20.0 g/L) Levulinic acid (1.0 g/L, initial and 3 times feeding) | 8.6 | 84 | 7.2 | 28 | 2.0 | 0.50 | [66] |
| C. necator H16 Fructose (20.0 g/L) Levulinic acid (3.5 g/L) | 7.3 | 48 | 3.5 | 16 | 0.6 | 0.16 | [11] |
| Hydrogenophaga pseudoflava DSM 1034 Whey permeate (47 mL/L) Levulinic acid (1.0 g/L) | 4.5 | 49 | 2.2 | 45 | 1.0 | 1.00 | [68] |
| Conjugate bases of organic acids | |||||||
| Caldimonas taiwanensis Sugars (1.5%) Valerate (0.5 g/L) | 1.6–4.1 | 42–67 | 0.8–2.1 | 10–13 | 0.1–0.2 | 0.16–0.49 | [78] |
| Methylocystis dominated mixed culture Methane gas (repeating 48 h fed-batch cycle) Valerate (0.4 g/L) | 1.5 | 30 | 0.5 | 39 | 0.2 | 0.45 | [79] |
| Sodium salts of organic acids | |||||||
| Azohydromonas lata Rice wastewater (21 g/L) Sodium acetate (10 g/L) | 5.0 | 32 | 1.6 | 6 | 0.1 | 0.01 | [80] |
| Corynebacterium glutamicum ATCC13869 transformant A Sodium propionate (1.0 g/L) | - | 31 | - | 28 | - | - | [81] |
| C. necator H16 Sodium acetate (0–20 g/L) Sodium propionate (0–20 g/L) | 0.3–0.7 | 12–56 | Trace | 0–45 | Trace | - | [82] |
| C. necator PHB−4 C Palm kernel oil (5.0 g/L) Sodium propionate (5.0 g/L) | 3.0 | 30 | 0.9 | 12 | 0.1 | 0.02 | [83] |
| Herbaspirillum seropedicae Z69Prp D Glucose (7.0 g/L) Sodium propionate (0.5 g/L) | 2.4 | 37 | 0.9 | 14 | 0.1 | 0.25 | [84] |
| C. necator H16 Plant oils (5.0 g/L) Sodium valerate (5.0 g/L) | 4.1–6.1 | 64–89 | 2.1–5.4 | 3–14 | 0.1–0.9 | 0.03–0.17 | [85] |
| C. necator PHB−4 C Palm kernel oil (5.0 g/L) Sodium valerate (1.0 g/L) | 4.2 | 52 | 2.2 | 6 | 0.1 | 0.13 | [83] |
| Methylocystis parvus OBB3 Methane gas (75 mL) Sodium valerate (1.0 g/L) | - | - | 0.3 | - | 0.2 | 0.20 | [86] |
| Alkyl alcohols | |||||||
| C. necator H16 Waste rapeseed oil (20.0 g/L) 1-propanol (8.0 g/L) | 14.7 | 80 | 11.7 | 9 | 1.1 | 0.14 | [13] |
| Erwinia sp. USMI-20 Palm oil (4.6 g/L) 1-propanol (2.3 g/L) | 5.4 | 50 | 2.7 | 6 | 0.2 | 0.07 | [60] |
| C. malaysiensis USMAA2-4 Oleic acid (6.5 g/L) 1-pentanol (0.9 g/L) | 5.1 | 40 | 2.1 | 8 | 0.2 | 0.22 | [87] |
| C. malaysiensis USMAA2-4ABH16 B Palm olein (6.5 g/L) 1-pentanol (0.9 g/L) | 5.4 | 69 | 3.7 | 7 | 0.3 | 0.33 | [87] |
| C. malaysiensis USMAA1020 Oleic acid (6.5 g/L) 1-pentanol (1.3 g/L) | - | 76 | - | 10 | - | - | [88] |
| Erwinia sp. USMI-20 Palm oil (4.6 g/L) 1-pentanol (1.4 g/L) | 4.8 | 62 | 3.0 | 20 | 0.6 | 0.43 | [60] |
| Massilia haematophila UMTKB-2 Glucose (16.0 g/L) 1-pentanol (1 g/L) | - | - | 5.0 | 7 | 0.4 | 0.40 | [89] |
| Methylobacterium extorquens G10 Methanol (fractional supply by 5–20 mL) 1-pentanol (fractional supply by 2%–20% v/v methanol) | 25–40 | 30–45 | 7.5–18.0 | 14–50 | 2.5–4.5 | - | [90] |
| Methylocystis sp. WRRC1 Methane gas (75 mL) 1-pentanol (1.0 g/L) | - | - | 0.3 | - | 0.2 | 0.17 | [86] |
| Methyloligella halotolerans C2 Methanol (5–20 mL fractional supply) 1-pentanol (fractional supply by 5–15% v/v methanol) | - | 49–98 | - | 2–51 | - | - | [91] |
| P. denitrificans ATCC 17741 1-pentanol (maintained at 1.6 g/L) | 6.8 | 18 | 1.2 | 100 | 1.2 | - | [14] |
| Mixed precursors | |||||||
| C. necator DSM 545 Levulinic acid (1.0 g/L) Sodium propionate (2.5 g/L) | 1.0 | 33 | 0.3 | 73 | 0.2 | 0.24 | [65] |
| C. necator DSM 545 Levulinic acid (1.0 g/L) Sodium propionate (1.0 g/L) | 0.5 | 19 | 0.1 | 78 | Trace | - | [10] |
| H. pseudoflava DSM 1034 Whey permeate (47.0 mL/L) Levulinic acid (0.5 g/L, initial and 3 times feeding) Sodium valerate (1.0 g/L, initial and 3 times feeding) | 6.6 | 67 | 4.4 | 55 | 2.4 | 0.43 | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, H.S.J.; Bhubalan, K.; Amirul, A.-A.A. A Critical Review on the Economically Feasible and Sustainable Poly(3-Hydroxybutyrate-co-3-hydroxyvalerate) Production from Alkyl Alcohols. Polymers 2022, 14, 670. https://doi.org/10.3390/polym14040670
Wong HSJ, Bhubalan K, Amirul A-AA. A Critical Review on the Economically Feasible and Sustainable Poly(3-Hydroxybutyrate-co-3-hydroxyvalerate) Production from Alkyl Alcohols. Polymers. 2022; 14(4):670. https://doi.org/10.3390/polym14040670
Chicago/Turabian StyleWong, Hau Seung Jeremy, Kesaven Bhubalan, and Al-Ashraf Abdullah Amirul. 2022. "A Critical Review on the Economically Feasible and Sustainable Poly(3-Hydroxybutyrate-co-3-hydroxyvalerate) Production from Alkyl Alcohols" Polymers 14, no. 4: 670. https://doi.org/10.3390/polym14040670