Facile Synthesis of Functionalised Hyperbranched Polymers for Application as Novel, Low Viscosity Lubricant Formulation Components
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Oil Soluble Hyperbranched Polymers
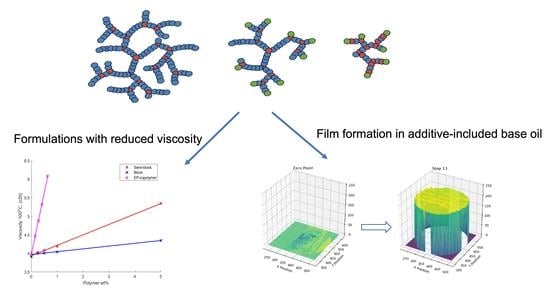
2.2. Application Testing of Controlled Morphology Hyperbranched Polymers
2.2.1. Viscosity
2.2.2. Film Formation
3. Conclusions
4. Experimental Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, A. The chemistry of lubricating oil additives. J. Chem. Educ. 1956, 33, 308. [Google Scholar] [CrossRef]
- Rudnick, L.R. Lubricant Additives: Chemistry and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- ATC. Lubricant Additives: Use and Benefits; Technical Committee of Petroleum Additive Manufacturers in Europe: Brussels, Belgium, 2016. [Google Scholar]
- Holmberg, K.; Andersson, P.; Erdemir, A. Global energy consumption due to friction in passenger cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Spikes, H. Friction Modifier Additives. Tribol. Lett. 2015, 60, 5. [Google Scholar] [CrossRef]
- Martini, A.; Ramasamy, U.S.; Len, M. Review of Viscosity Modifier Lubricant Additives. Tribol. Lett. 2018, 66, 58. [Google Scholar] [CrossRef]
- Cosimbescu, L.; Robinson, J.W.; Page, J.P. Polymer Architecture: Does It Influence Shear Stability? Ind. Eng. Chem. Res 2018, 57, 12. [Google Scholar] [CrossRef]
- Mortier, R.M.; Orszulik, S.T. Chemistry and Technology of Lubricants; Springer: Dordrecht, The Netherlands, 1994; ISBN 1461535549. [Google Scholar]
- Hong, F.T.; Ladelta, V.; Gautam, R.; Sarathy, S.M.; Hadjichristidis, N. Polyether-Based Block Co(ter)polymers as Multifunctional Lubricant Additives. ACS Appl. Polym. Mater. 2021, 3, 3811–3820. [Google Scholar] [CrossRef]
- Campbell, K.B.; Erck, R.; Swita, M.; Cosimbescu, L. Multifunctional Tunable Polymethacrylates for Enhanced Shear Stability and Wear Prevention. Cite This ACS Appl. Polym. Mater 2020, 2, 2839–2848. [Google Scholar] [CrossRef]
- Winer, W.O. Molybdenum disulfide as a lubricant: A review of the fundamental knowledge. Wear 1967, 10, 422–452. [Google Scholar] [CrossRef]
- Cizaire, L.; Vacher, B.; Le Mogne, T.; Martin, J.M.; Rapoport, L.; Margolin, A.; Tenne, R. Mechanisms of ultra-low friction by hollow inorganic fullerene-like MoS2 nanoparticles. Surf. Coat. Technol. 2002, 160, 282–287. [Google Scholar] [CrossRef]
- Wiesbrock, F.; Hoogenboom, R.; Schubert, U.S. Microwave-assisted polymer synthesis: State-of-the-art and future perspectives. Macromol. Rapid Commun. 2004, 25, 1739–1764. [Google Scholar] [CrossRef]
- Nunez, C.M.; Chiou, B.S.; Andrady, A.L.; Khan, S.A. Solution rheology of hyperbranched polyesters and their blends with linear polymers. Macromolecules 2000, 33, 1720–1726. [Google Scholar] [CrossRef]
- Buhleier, E.; Wehner, W.; Vogtle, F. “Cascade”- and “Nonskid-Chain-like” Syntheses of Molecular Cavity Topologies. Synthesis 1978, 2, 155–158. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Newkome, G.R.; YaolC, Z.; Baker, G.R.; Gupta, V.K. Cascade Molecules: A New Approach to Micelles. A [27]-Arborol. J. Org. Chem. 1985, 50, 2003–2004. [Google Scholar] [CrossRef]
- Lee, C.C.; Mackay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef]
- Crooks, R.M.; Zhao, M.; Sun, L.; Chechik, V.; Yeung, L.K. Dendrimer-Encapsulated Metal Nanoparticles: Synthesis, Characterization, and Applications to Catalysis. Acc. Chem. Res. 2001, 34, 181–190. [Google Scholar] [CrossRef]
- Freeman, A.W.; Koene, S.C.; Malenfant, P.R.L.; Thompson, M.E.; Fréchet, J.M.J. Dendrimer-containing light-emitting diodes: Toward site-isolation of chromophores. J. Am. Chem. Soc. 2000, 122, 12385–12386. [Google Scholar] [CrossRef]
- Ihre, H.R.; Padilla De Jesus, O.L.; Szoka, F.C., Jr.; Frechet, J.M. Polyester dendritic systems for drug delivery applications: Design, synthesis, and characterization. Bioconjug. Chem. 2002, 13, 443–452. [Google Scholar] [CrossRef]
- Kil, S.B.; Augros, Y.; Leterrier, Y.; Mhison, J.-A.E.; Christel, A.; Borer, C. Rheological Properties of Hyperbranched Polymer/Poly(ethylene Terephthalate) Reactive Blends. Polym. Eng. Sci. 2003, 43, 329–343. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef]
- Inoue, K. Functional dendrimers, hyperbranched and star polymers. Prog. Polym. Sci. 2000, 25, 453–571. [Google Scholar] [CrossRef]
- Gao, C.; Yan, D.; Frey, H. Promising Dendritic Materials: An Introduction to Hyperbranched Polymers. In Hyperbranched Polymers: Synthesis, Properties, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Kim, K.-M.; Jikei, M.; Kakimoto, M. Preparation and Properties of Novel Hyperbranched Poly(dimethylsiloxane)s. Polym. J. 2002, 34, 275–279. [Google Scholar] [CrossRef]
- Kienle, R.H.; van der Meulen, P.A.; Petke, F.E. The Polyhydric Alcohol-Polybasic Acid Reaction. IV. Glyceryl Phthalate from Phthalic Acid. J. Am. Chem. Soc. 1939, 61, 2268–2271. [Google Scholar] [CrossRef]
- Kim, Y.H. Hyperbranched polyarylene. US Patent US4857630A, 15 August 1989. [Google Scholar]
- Cassin, S.R.; Flynn, S.; Chambon, P.; Rannard, S.P. Accessing new and scalable high molecular weight branched copolymer structures using transfer-dominated branching radical telomerisation (TBRT). Polym. Chem. 2022, 13, 2295–2306. [Google Scholar] [CrossRef]
- Cassin, S.R.; Flynn, S.; Chambon, P.; Rannard, S.P. Quantification of branching within high molecular weight polymers with polyester backbones formed by transfer-dominated branching radical telomerisation (TBRT). RSC Adv. 2021, 11, 24374–24380. [Google Scholar] [CrossRef]
- Zheng, C.; Fu, H.; Wang, Y.; Zhang, T.; Huang, Z.; Duan, W. Preparation and mechanism of hyperbranched heavy oil viscosity reducer. J. Pet. Sci. Eng. 2021, 196, 107941. [Google Scholar] [CrossRef]
- Karmakar, G.; Dey, K.; Ghosh, P.; Sharma, B.K.; Erhan, S.Z. A short review on polymeric biomaterials as additives for lubricants. Polymers 2021, 13, 1333. [Google Scholar] [CrossRef]
- Robinson, J.W.; Zhou, Y.; Bhattacharya, P.; Erck, R.; Qu, J.; Bays, J.T.; Cosimbescu, L. Probing the molecular design of hyper-branched aryl polyesters towards lubricant applications. Sci. Rep. 2016, 6, 18624. [Google Scholar] [CrossRef]
- Robinson, J.W.; Zhou, Y.; Qu, J.; Bays, J.T.; Cosimbescu, L. Highly branched polyethylenes as lubricant viscosity and friction modifiers. React. Funct. Polym. 2016, 109, 52–55. [Google Scholar] [CrossRef]
- Li, X.; Shi, L.; Li, H.; Liu, P.; Luo, J.; Yuan, Z. Experimental study on viscosity reducers for SAGD in developing extra-heavy oil reservoirs. J. Pet. Sci. Eng. 2018, 166, 25–32. [Google Scholar] [CrossRef]
- Quan, H.; Chen, L.; Huang, Z.; Wang, J.; Zheng, C. The effect of a kind of hyperbranched polyester with different carbon length on flowability for crude oil. Fuel 2018, 214, 356–362. [Google Scholar] [CrossRef]
- Sun, H.; Lei, X.; Shen, B.; Zhang, H.; Liu, J.; Li, G.; Wu, D. Rheological properties and viscosity reduction of South China Sea crude oil. J. Energy Chem. 2018, 27, 1198–1207. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.; Cheng, Z.; Wu, W.; Zhang, J.; Zhang, J.; Yang, Z.; Zhang, D. New Composite Viscosity Reducer with Both Asphaltene Dispersion and Emulsifying Capability for Heavy and Ultraheavy Crude Oils. Energy Fuels 2017, 31, 1159–1173. [Google Scholar] [CrossRef]
- Mao, J.; Liu, J.; Peng, Y.; Zhang, Z.; Zhao, J. Quadripolymers as Viscosity Reducers for Heavy Oil. Energy Fuels 2018, 32, 119–124. [Google Scholar] [CrossRef]
- Du, Q.; Liu, H.; Wu, G.; Hou, J.; Zhou, K.; Liu, Y. Application of flue-gas foam in thermal-chemical flooding for medium-depth heavy oil reservoirs. Energy Sci. Eng. 2019, 7, 2936–2949. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, S.; Huang, C.; Tian, Y.; Yang, X.; Jing, X.; Li, A. Screening of Synthesis Conditions of Polymers and the Effects on Viscosity Reduction Performance. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Banda Aceh, Indonesia, 26–27 September 2018; IOP Publishing: Bristol, UK, 2018; Volume 108. [Google Scholar]
- Mao, J.; Liu, J.; Wang, H.; Yang, X.; Zhang, Z.; Yang, B.; Zhao, J. Novel terpolymers as viscosity reducing agent for Tahe super heavy oil. RSC Adv. 2017, 7, 19257–19261. [Google Scholar] [CrossRef]
- Shahsavar, N.; Riazi, M.; Malayeri, M.R. Removal of asphaltene deposition in porous media using emulsified solvents—A visual study. J. Pet. Sci. Eng. 2020, 191, 107207. [Google Scholar] [CrossRef]
- Gao, X.L.; Wang, C.F.; Feng, J.Z.; Sun, Q.; Xue, L.Q.; Ma, W.N.; Wang, H.; Su, N.; Liu, G.Y.; Liu, L.F.; et al. Application of compound viscosity reducer for low temperature transportation in Huabei oilfield. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Sanya, China, 17–19 November 2018; IOP Publishing: Bristol, UK, 2019; Volume 479. [Google Scholar]
- Sun, J.H.; Zhang, F.S.; Wu, Y.W.; Liu, G.L.; Li, X.N.; Su, H.M.; Zhu, Z.Y. Overview of emulsified viscosity reducer for enhancing heavy oil recovery. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Sanya, China, 17–19 November 2018; IOP Publishing: Bristol, UK, 2019; Volume 479. [Google Scholar]
- Costello, P.; Martin, I.; Slark, A.; Sherrington, D.; Titterton, A. Branched methacrylate copolymers from multifunctional monomers: Chemical composition and physical architecture distributions. Polymer 2002, 43, 245–254. [Google Scholar] [CrossRef]
- O’Brien, N.; McKee, A.; Sherrington, D.C.; Slark, A.T.; Titterton, A. Facile, versatile and cost effective route to branched vinyl polymers. Polymer 2000, 41, 6027–6031. [Google Scholar] [CrossRef]
- Guan, Z. Control of Polymer Topology through Metal Catalysis: Synthesis of Hyperbranched Polymers by Cobalt-Mediated Free Radical Polymerization. J. Am. Chem. Soc. 2002, 124, 5616–5617. [Google Scholar] [CrossRef] [PubMed]
- Haddleton, D.M.; Maloney, D.R.; Suddaby, K.G. Catalytic chain transfer polymerisation (CCTP) of methyl methacrylate: Effect of catalyst structure and reaction conditions on chain transfer coefficient. Macromol. Symp. 1996, 111, 37–46. [Google Scholar] [CrossRef]
- Adlington, K.; Irvine, D.J.; Barker, I.; Stevens, L. Accelerated Synthesis of Hyperbranched Divinylbenzene Polymers via Catalytic Chain Transfer Polymerisation and their Use as Gas Capture Agents. In Manuscript in Preparation; University of Nottingham: Nottingham, UK, 2017. [Google Scholar]
- Suddaby, K.G.; Maloney, D.R.; Haddleton, D.M. Homopolymerizations of methyl methacrylate and styrene: Chain transfer constants from the Mayo equation and number distributions for catalytic chain transfer, and the chain length dependence of the average termination rate coefficient. Macromolecules 1997, 30, 702–713. [Google Scholar] [CrossRef]
- Barker, I.A.; Harfi, J.E.; Adlington, K.; Howdle, S.M.; Irvine, D.J. Catalytic Chain Transfer Mediated Autopolymerization of Divinylbenzene: Toward Facile Synthesis of High Alkene Functional Group Density Hyperbranched Materials. Macromolecules 2012, 45, 9258–9266. [Google Scholar] [CrossRef]
- Haddleton, D.M.; Maloney, D.R.; Suddaby, K.G. Competition between β-Scission of Macromonomer-Ended Radicals and Chain Transfer to Cobalt(II) in Catalytic Chain Transfer Polymerization (CCTP). Macromolecules 1996, 29, 481–483. [Google Scholar] [CrossRef]
- Ivan, B. Terminology and Classification of Quasiliving Polymerizations and Ideal Living Polymerizations on the Basis of the Logic of Elementary Polymerization Reactions, and Comments on Using the Term “Controlled”. Macromol. Chem. Phys 2000, 201, 2621–2628. [Google Scholar]
- Kabir, S.F.K.; Adlington, K.; Parsons, A.; Ahmed, I.; Irvine, D. Comparison of thermal, thermomechanical, and rheological properties of blends of divinylbenzene-based hyperbranched and linear functionalized polymers. J. Appl. Polym. Sci. 2019, 137, 48547. [Google Scholar] [CrossRef]
- Szanka, A.; Szarka, G.; Iván, B. Poly(methyl methacrylate-co-2-hydroxyethyl methacrylate) four-arm star functional copolymers by quasiliving ATRP: Equivalent synthetic routes by protected and nonprotected HEMA comonomers. J. Macromol. Sci. Part A Pure Appl. Chem. 2014, 51, 125–133. [Google Scholar] [CrossRef]
- Szanka, A.; Szarka, G.; Iván, B. Multi-methacrylated star-shaped, photocurable poly(methyl methacrylate) macromonomers via quasiliving ATRP with suppressed curing shrinkage. Polymer 2013, 54, 6073–6077. [Google Scholar] [CrossRef]
- Kamaruddin, M.J.; Nguyen, N.T.; Dimitrakis, G.A.; El, J.; Binner, E.R.; Kingman, S.W.; Lester, E.; Robinson, J.P.; Irvine, D.J. Continuous and direct “in situ” reaction monitoring of chemical reactions via dielectric property measurement: Controlled polymerisation. RSC Adv. 2014, 4, 5709–5717. [Google Scholar] [CrossRef]
- Adlington, K.; Nguyen, N.T.; Eaves, E.; Yang, J.; Chang, C.-Y.; Li, J.; Gower, A.L.; Stimpson, A.; Anderson, D.G.; Langer, R.; et al. Application of Targeted Molecular and Material Property Optimization to Bacterial Attachment-Resistant (Meth)acrylate Polymers. Biomacromolecules 2016, 17, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Adlington, K.; Jones, G.J.; Harfi, J.E.; Dimitrakis, G.; Smith, A.; Kingman, S.W.; Robinson, J.P.; Irvine, D.J. Mechanistic Investigation into the Accelerated Synthesis of Methacrylate Oligomers via the Application of Catalytic Chain Transfer Polymerization and Selective Microwave Heating. Macromolecules 2013, 46, 3922–3930. [Google Scholar] [CrossRef]
- Gaborieau, M.; Castignolles, P. Size-exclusion chromatography (SEC) of branched polymers and polysaccharides. Anal. Bioanal. Chem. 2011, 399, 1413–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Comonomer | Solvation Temp (°C) | Appearance in Solution |
|---|---|---|
| SA | 70 | Colloidal Suspension |
| SMA | 70 | Clear Solution |
| LA a | 50 | Clear Solution |
| LMA | 50 | Clear Solution |
| Entry | Structure | CTA Oligomer Used | Time (Min) | Yield a (%) | Mn b (g mol−1) | Đ b |
|---|---|---|---|---|---|---|
| 1 | Random | PhCoBF | 35 | 53 | 3650 | 4.9 |
| 2 | Semi-Block-like | Crude LMA | 35 | 66 | 3700 | 102.7 |
| 3 | Block-like | Purified LMA | 35 | 65 | 4400 | 58.4 |
| 4 | Block like | Purified LMA | 10 | na c | 2500 | 5.5 |
| 5 | Block like | Purified LMA | 20 | na c | 3300 | 6.7 |
| 6 | Block-like | Purified LMA | 30 | na c | 4200 | 74.7 |
| 7 | Block-like | Purified LMA | 40 | Gel | Gel | Gel |
| Step No | Maximum Thickness [nm] | Minimum Thickness [nm] | Mean Thickness [nm] | Standard Deviation [nm] | Measurement Temperature [°C] |
|---|---|---|---|---|---|
| A (Zero Point) | 5.0 | −10.0 | 0.3 | 1.5 | 60 |
| B (Step 3) | 241.0 | −10.0 | 71.1 | 89.4 | 60 |
| C (Step 5) | 233.0 | −6.0 | 54.0 | 83.0 | 60 |
| D (Step 7) | 240.0 | −10.0 | 4.5 | 19.8 | 90 |
| Step No | Maximum Thickness [nm] | Minimum Thickness [nm] | Mean Thickness [nm] | Standard Deviation [nm] | Measurement Temperature [°C] |
|---|---|---|---|---|---|
| A (Zero Point) | 4.0 | −10.0 | 0.2 | 1.3 | 60 |
| B (Step 3) | 217.0 | 0.0 | 179.9 | 67.9 | 60 |
| C (Step 5) | 231.0 | 0.0 | 184.3 | 63.0 | 60 |
| D (Step 7) | 241.0 | 7.0 | 196.3 | 53.6 | 90 |
| E (Step 9) | 230.0 | 0.0 | 200.3 | 35.4 | 90 |
| F (Step 11) | 216.0 | 127.0 | 202.3 | 3.4 | 120 |
| G (Step13) | 219.0 | 74.0 | 202.3 | 4.2 | 120 |
| Step No | Maximum Thickness [nm] | Minimum Thickness [nm] | Mean Thickness [nm] | Standard Deviation [nm] | Measurement Temperature [°C] |
|---|---|---|---|---|---|
| A (Zero Point) | 6.0 | −10.0 | 0.7 | 1.7 | 60 |
| B (Step 3) | 218.0 | −3.0 | 194.9 | 44.6 | 60 |
| C (Step 5) | 227.0 | 1.0 | 194.4 | 46.6 | 60 |
| D (Step 7) | 223.0 | 80.0 | 200.1 | 12.1 | 90 |
| E (Step 9) | 227.0 | 118.0 | 201.0 | 9.9 | 90 |
| F (Step 11) | 223.0 | 75.0 | 204.4 | 8.8 | 120 |
| G (Step13) | 220.0 | 118.0 | 201.4 | 13.3 | 120 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goodwin, S.R.; Stimpson, A.; Moon, R.; Cowie, L.; Aragrag, N.; Filip, S.V.; Smith, A.G.; Irvine, D.J. Facile Synthesis of Functionalised Hyperbranched Polymers for Application as Novel, Low Viscosity Lubricant Formulation Components. Polymers 2022, 14, 3841. https://doi.org/10.3390/polym14183841
Goodwin SR, Stimpson A, Moon R, Cowie L, Aragrag N, Filip SV, Smith AG, Irvine DJ. Facile Synthesis of Functionalised Hyperbranched Polymers for Application as Novel, Low Viscosity Lubricant Formulation Components. Polymers. 2022; 14(18):3841. https://doi.org/10.3390/polym14183841
Chicago/Turabian StyleGoodwin, Sophie R., Amy Stimpson, Richard Moon, Lauren Cowie, Najib Aragrag, Sorin V. Filip, Andrew G. Smith, and Derek J. Irvine. 2022. "Facile Synthesis of Functionalised Hyperbranched Polymers for Application as Novel, Low Viscosity Lubricant Formulation Components" Polymers 14, no. 18: 3841. https://doi.org/10.3390/polym14183841