MoS2-Cu/CuO@graphene Heterogeneous Photocatalysis for Enhanced Photocatalytic Degradation of MB from Water
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of MoS2-Cu/CuO@GN
2.3. Photodegradation Measurement
2.4. Characterizations
3. Results and Discussion
3.1. Structural Analysis
3.2. Optical Properties
3.3. Surface Compositional Analysis
3.4. Surface Morphology
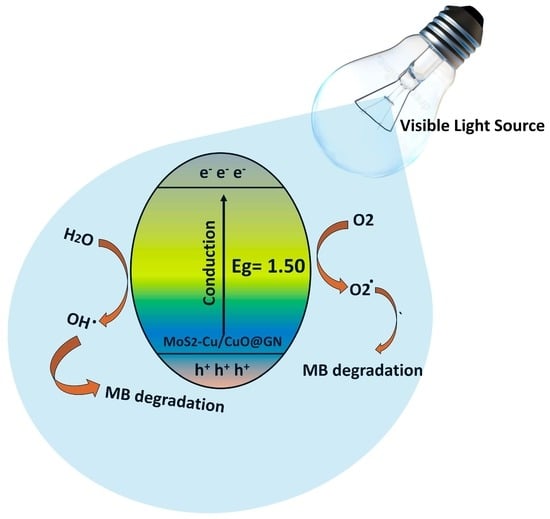
3.5. Photocatalytic Activity
3.5.1. Effect of Reaction Parameter
Effect of MoS2-Cu/CuO@GN Dosage
Effect of Hydrogen Peroxide
Reusability of MoS2-Cu/CuO@GN
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakraborty, J.; Nath, I.; Verpoort, F. A physicochemical introspection of porous organic polymer photocatalysts for wastewater treatment. Chem. Soc. Rev. 2022, 51, 1124–1138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, S.; Li, A.; Liu, D.; Sun, H.; Zhao, F. Low-cost bauxite residue-MoS2 possessing adsorption and photocatalysis ability for removing organic pollutants in wastewater. Sep. Purif. Technol. 2022, 283, 120144. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Mohana Roopan, S.; Khan, M.A. MoS2 based ternary composites: Review on heterogeneous materials as catalyst for photocatalytic degradation. Catal. Rev. 2021, 1–74. [Google Scholar] [CrossRef]
- Gautam, S.; Agrawal, H.; Thakur, M.; Akbari, A.; Sharda, H.; Kaur, R.; Amini, M. Metal oxides and metal organic frameworks for the photocatalytic degradation: A review. J. Environ. Chem. Eng. 2020, 8, 103726. [Google Scholar] [CrossRef]
- Heuer, J.; Ferguson, C.T.J. Photocatalytic polymer nanomaterials for the production of high value compounds. Nanoscale 2022, 14, 1646–1652. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Ramakrishnan, S.; Park, B.-H.; Han, M.-K.; Yoo, D.J. Enhanced electrochemical performance and long-term durability of composite membranes through a binary interface with sulfonated unzipped graphite nanofibers for polymer electrolyte fuel cells operating under low relative humidity. Appl. Surf. Sci. 2022, 593, 153407. [Google Scholar] [CrossRef]
- Rani, A.; Singh, K.; Sharma, P. Investigation of visible light photocatalytic degradation of organic dyes by MoS2 nanosheets synthesized by different routes. Bull. Mater. Sci. 2022, 45, 63. [Google Scholar] [CrossRef]
- Lai, M.T.L.; Lee, K.M.; Yang, T.C.K.; Pan, G.T.; Lai, C.W.; Chen, C.-Y.; Johan, M.R.; Juan, J.C. The improved photocatalytic activity of highly expanded MoS2 under visible light emitting diodes. Nanoscale Adv. 2021, 3, 1106–1120. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Ezzat, H.A.; Yahia, I.S.; Zahran, H.Y.; Elhaes, H.; Gomaa, I.; Ibrahim, M.A. Effect of CuO and Graphene on PTFE Microfibers: Experimental and Modeling Approaches. Polymers 2022, 14, 1069. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, J.; Ma, Q.; Ma, J.; Bai, H.; Chen, L.; Mu, S. Two-Dimensional MoS2: Structural Properties, Synthesis Methods, and Regulation Strategies toward Oxygen Reduction. Micromachines 2021, 12, 240. [Google Scholar] [CrossRef]
- Li, H.; Yu, K.; Lei, X.; Guo, B.; Li, C.; Fu, H.; Zhu, Z. Synthesis of the MoS2@CuO heterogeneous structure with improved photocatalysis performance and H2O adsorption analysis. Dalton Trans. 2015, 44, 10438–10447. [Google Scholar] [CrossRef]
- Ashar, A.; Bhatti, I.A.; Jilani, A.; Mohsin, M.; Rasul, S.; Iqbal, J.; Shakoor, M.B.; Al-Sehemi, A.G.; Wageh, S.; Al-Ghamdi, A.A. Enhanced Solar Photocatalytic Reduction of Cr(VI) Using a (ZnO/CuO) Nanocomposite Grafted onto a Polyester Membrane for Wastewater Treatment. Polymers 2021, 13, 4047. [Google Scholar] [CrossRef]
- Xu, L.; Srinivasakannan, C.; Peng, J.; Zhang, L.; Zhang, D. Synthesis of Cu-CuO nanocomposite in microreactor and its application to photocatalytic degradation. J. Alloys Compd. 2017, 695, 263–269. [Google Scholar] [CrossRef]
- Jinfeng, Z.; Yunguang, Y.; Wei, L. Preparation, Characterization, and Activity Evaluation of CuO/F-TiO2 Photocatalyst. Int. J. Photoenergy 2012, 2012, 139739. [Google Scholar] [CrossRef] [Green Version]
- Jilani, A.; Othman, M.H.D.; Ansari, M.O.; Hussain, S.Z.; Ismail, A.F.; Khan, I.U.; Inamuddin. Graphene and its derivatives: Synthesis, modifications, and applications in wastewater treatment. Environ. Chem. Lett. 2018, 16, 1301–1323. [Google Scholar] [CrossRef]
- Albero, J.; Mateo, D.; García, H. Graphene-Based Materials as Efficient Photocatalysts for Water Splitting. Molecules 2019, 24, 906. [Google Scholar] [CrossRef] [Green Version]
- Saeed, U.; Jilani, A.; Iqbal, J.; Al-Turaif, H. Reduced graphene oxide-assisted graphitic carbon nitride@ZnO rods for enhanced physical and photocatalytic degradation. Inorg. Chem. Commun. 2022, 142, 109623. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, N.; Opoku, F.; Govender, P.P.; Ray, S.S. MoS2 Nanosheet/ZnS Composites for the Visible-Light-Assisted Photocatalytic Degradation of Oxytetracycline. ACS Appl. Nano Mater. 2021, 4, 4721–4734. [Google Scholar] [CrossRef]
- Trang Phan, T.T.; Truong, T.T.; Huu, H.T.; Nguyen, L.T.; Nguyen, V.T.; Nguyen, H.L.; Vo, V. Visible Light-Driven Mn-MoS2/rGO Composite Photocatalysts for the Photocatalytic Degradation of Rhodamine B. J. Chem. 2020, 2020, 6285484. [Google Scholar] [CrossRef]
- Jilani, A.; Othman, M.H.D.; Ansari, M.O.; Kumar, R.; Alshahrie, A.; Ismail, A.F.; Khan, I.U.; Sajith, V.K.; Barakat, M.A. Facile spectroscopic approach to obtain the optoelectronic properties of few-layered graphene oxide thin films and their role in photocatalysis. New J. Chem. 2017, 41, 14217–14227. [Google Scholar] [CrossRef]
- Khan, A.; Rashid, A.; Younas, R.; Chong, R. A chemical reduction approach to the synthesis of copper nanoparticles. Int. Nano Lett. 2016, 6, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Dustgeer, M.R.; Asma, S.T.; Jilani, A.; Raza, K.; Hussain, S.Z.; Shakoor, M.B.; Iqbal, J.; Abdel-wahab, M.S.; Darwesh, R. Synthesis and characterization of a novel single-phase sputtered Cu2O thin films: Structural, antibacterial activity and photocatalytic degradation of methylene blue. Inorg. Chem. Commun. 2021, 128, 108606. [Google Scholar] [CrossRef]
- Mudassir, M.A.; Hussain, S.Z.; Jilani, A.; Zhang, H.; Ansari, T.M.; Hussain, I. Magnetic Hierarchically Macroporous Emulsion-Templated Poly(acrylic acid)–Iron Oxide Nanocomposite Beads for Water Remediation. Langmuir 2019, 35, 8996–9003. [Google Scholar] [CrossRef]
- Mishra, S.K.; Tripathi, S.N.; Choudhary, V.; Gupta, B.D. SPR based fibre optic ammonia gas sensor utilizing nanocomposite film of PMMA/reduced graphene oxide prepared by in situ polymerization. Sens. Actuators B Chem. 2014, 199, 190–200. [Google Scholar] [CrossRef]
- Gascho, J.L.S.; Costa, S.F.; Recco, A.A.C.; Pezzin, S.H. Graphene Oxide Films Obtained by Vacuum Filtration: X-ray Diffraction Evidence of Crystalline Reorganization. J. Nanomater. 2019, 2019, 5963148. [Google Scholar] [CrossRef] [Green Version]
- Jilani, A.; Hussain, S.Z.; Melaibari, A.A.; Abu-Hamdeh, N.H. Development and Mechanistic Studies of Ternary Nanocomposites for Hydrogen Production from Water Splitting to Yield Sustainable/Green Energy and Environmental Remediation. Polymers 2022, 14, 1290. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Zaen, R.; Oktiani, R. Correlation between crystallite size and photocatalytic performance of micrometer-sized monoclinic WO3 particles. Arab. J. Chem. 2020, 13, 1283–1296. [Google Scholar] [CrossRef]
- Jilani, A.; Abdel-wahab, M.S.; Al-ghamdi, A.A.; Dahlan, A.s.; Yahia, I.S. Nonlinear optical parameters of nanocrystalline AZO thin film measured at different substrate temperatures. Phys. B Condens. Matter 2016, 481, 97–103. [Google Scholar] [CrossRef]
- Jilani, A.; Othman, M.H.D.; Ansari, M.O.; Oves, M.; Alshahrie, A.; Khan, I.U.; Sajith, V. A simple route to layer-by-layer assembled few layered graphene oxide nanosheets: Optical, dielectric and antibacterial aspects. J. Mol. Liq. 2018, 253, 284–296. [Google Scholar] [CrossRef]
- Jeong, D.; Jo, W.; Jeong, J.; Kim, T.; Han, S.; Son, M.-K.; Jung, H. Characterization of Cu2O/CuO heterostructure photocathode by tailoring CuO thickness for photoelectrochemical water splitting. RSC Advances 2022, 12, 2632–2640. [Google Scholar] [CrossRef]
- Gopal, R.; Chinnapan, M.M.; Bojarajan, A.K.; Rotte, N.K.; Ponraj, J.S.; Ganesan, R.; Atanas, I.; Nadarajah, M.; Manavalan, R.K.; Gaspar, J. Facile synthesis and defect optimization of 2D-layered MoS2 on TiO2 heterostructure for industrial effluent, wastewater treatments. Sci. Rep. 2020, 10, 21625. [Google Scholar] [CrossRef]
- Mondal, A.; Prabhakaran, A.; Gupta, S.; Subramanian, V.R. Boosting Photocatalytic Activity Using Reduced Graphene Oxide (RGO)/Semiconductor Nanocomposites: Issues and Future Scope. ACS Omega 2021, 6, 8734–8743. [Google Scholar] [CrossRef]
- Jilani, A.; Othman, M.H.D.; Ansari, M.O.; Oves, M.; Hussain, S.Z.; Khan, I.U.; Abdel-wahab, M.S. Structural and optical characteristics, and bacterial decolonization studies on non-reactive RF sputtered Cu–ZnO@graphene based nanoparticles thin films. J. Mater. Sci. 2019, 54, 6515–6529. [Google Scholar] [CrossRef]
- Wang, B.; Yang, S.; Chen, J.; Mann, C.; Bushmaker, A.; Cronin, S.B. Radiation-induced direct bandgap transition in few-layer MoS2. Appl. Phys. Lett. 2017, 111, 131101. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Jit, S.; Tripathi, S. MoS2, rGO, and CuO Nanocomposite-Based High Performance UV-Visible Dual-Band Photodetectors. IEEE Photonics Technol. Lett. 2021, 33, 93–96. [Google Scholar] [CrossRef]
- Abid; Sehrawat, P.; Islam, S.S.; Mishra, P.; Ahmad, S. Reduced graphene oxide (rGO) based wideband optical sensor and the role of Temperature, Defect States and Quantum Efficiency. Sci. Rep. 2018, 8, 3537. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Yang, S.; He, L.; Ye, C.; Song, X.; Liao, F. Quantum size effect of poly(o-phenylenediamine) quantum dots: From controllable fabrication to tunable photoluminescence properties. Synth. Met. 2014, 198, 142–149. [Google Scholar] [CrossRef]
- Muralikrishna, S.; Sureshkumar, K.; Varley, T.S.; Nagaraju, D.H.; Ramakrishnappa, T. In situ reduction and functionalization of graphene oxide with l-cysteine for simultaneous electrochemical determination of cadmium(ii), lead(ii), copper(ii), and mercury(ii) ions. Anal. Methods 2014, 6, 8698–8705. [Google Scholar] [CrossRef]
- Zhang, T.; He, Y.; Wang, F.; Li, H.; Duan, C.; Wu, C. Surface analysis of cobalt-enriched crushed products of spent lithium-ion batteries by X-ray photoelectron spectroscopy. Sep. Purif. Technol. 2014, 138, 21–27. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics Division, Perkin-Elmer Corporation: Chanhassen, MN, USA, 1992. [Google Scholar]
- Cheng, C.-K.; Lin, C.-H.; Wu, H.-C.; Ma, C.-C.M.; Yeh, T.-K.; Chou, H.-Y.; Tsai, C.-H.; Hsieh, C.-K. The Two-Dimensional Nanocomposite of Molybdenum Disulfide and Nitrogen-Doped Graphene Oxide for Efficient Counter Electrode of Dye-Sensitized Solar Cells. Nanoscale Res. Lett. 2016, 11, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinirtaş Ilkme, E.; Pozan Soylu, G.S. The role of some metal ions in enhancement of photocatalytic activity of Fe(2)O(3)-V(2)O(5) binary oxide. Turk. J. Chem. 2021, 45, 348–361. [Google Scholar] [CrossRef]
- Giubileo, F.; Grillo, A.; Passacantando, M.; Urban, F.; Iemmo, L.; Luongo, G.; Pelella, A.; Loveridge, M.; Lozzi, L.; Di Bartolomeo, A. Field Emission Characterization of MoS2 Nanoflowers. Nanomaterials 2019, 9, 717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, X.; Niu, L.; Song, L.; Wang, X.; Shi, X.; Yan, J. Effect of Polymer Addition on the Structure and Hydrogen Evolution Reaction Property of Nanoflower-Like Molybdenum Disulfide. Metals 2015, 5, 1829–1844. [Google Scholar] [CrossRef] [Green Version]
- Kaur, J.; Vergara, A.; Rossi, M.; Gravagnuolo, A.M.; Valadan, M.; Corrado, F.; Conte, M.; Gesuele, F.; Giardina, P.; Altucci, C. Electrostatically driven scalable synthesis of MoS2–graphene hybrid films assisted by hydrophobins. RSC Adv. 2017, 7, 50166–50175. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tang, X.; Liu, Z.; Liu, Z.; Yan, Y.; Yang, B.; Zhu, Z. Fabrication of a Z-scheme MoS2/CuO heterojunction for enhanced 2-mercaptobenzothiazole degradation activity and mechanism insight. New J. Chem. 2020, 44, 18264–18273. [Google Scholar] [CrossRef]
- Li, Z.; Pi, Y.; Xu, D.; Li, Y.; Peng, W.; Zhang, G.; Zhang, F.; Fan, X. Utilization of MoS2 and graphene to enhance the photocatalytic activity of Cu2O for oxidative CC bond formation. Appl. Catal. B Environ. 2017, 213, 1–8. [Google Scholar] [CrossRef]
- Zhong, X.; Royer, S.; Zhang, H.; Huang, Q.; Xiang, L.; Valange, S.; Barrault, J. Mesoporous silica iron-doped as stable and efficient heterogeneous catalyst for the degradation of C.I. Acid Orange 7 using sono–photo-Fenton process. Sep. Purif. Technol. 2011, 80, 163–171. [Google Scholar] [CrossRef]









| Material Details | Grain Size (nm) | Dislocation | Lattice Strain | Cell Volume Only for MoS2 |
|---|---|---|---|---|
| MoS2 | 2.37 | 4.46 × 10−1 | 2.01 × 10−2 | 10.84 |
| MoS2-Cu/CuO | 13.05 | 1.81 × 10−2 | 4.00 × 10−3 | 10.96 |
| MoS2-Cu/CuO@GN | 18.27 | 1.96 × 10−2 | 3.79 × 10−3 | 10.47 |
| Sample Detail | Detected Element (%) | ||||
|---|---|---|---|---|---|
| S2p | O1s | Mo3d | Cu2P3 | C1s | |
| MoS2 | 43.9 | 30.6 | 25.5 | --- | ---- |
| MoS2 Cu/CuO | 30.5 | 42.1 | 18.4 | 8.9 | ---- |
| MoS2 Cu/CuO@GN | 10.6 | 28.9 | 6.0 | 7.8 | 46.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jilani, A.; Melaibari, A.A. MoS2-Cu/CuO@graphene Heterogeneous Photocatalysis for Enhanced Photocatalytic Degradation of MB from Water. Polymers 2022, 14, 3259. https://doi.org/10.3390/polym14163259
Jilani A, Melaibari AA. MoS2-Cu/CuO@graphene Heterogeneous Photocatalysis for Enhanced Photocatalytic Degradation of MB from Water. Polymers. 2022; 14(16):3259. https://doi.org/10.3390/polym14163259
Chicago/Turabian StyleJilani, Asim, and Ammar A. Melaibari. 2022. "MoS2-Cu/CuO@graphene Heterogeneous Photocatalysis for Enhanced Photocatalytic Degradation of MB from Water" Polymers 14, no. 16: 3259. https://doi.org/10.3390/polym14163259