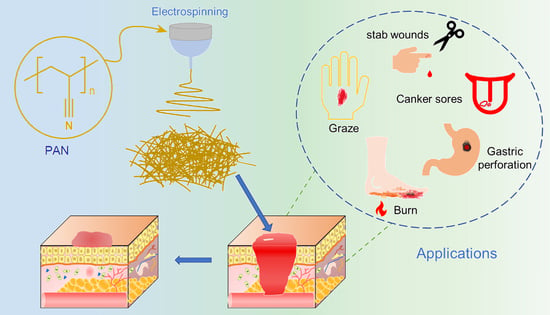
Recent Progress in Electrospun Polyacrylonitrile Nanofiber-Based Wound Dressing
Abstract
:1. Introduction
2. Wound Healing Process and Ideal Wound Care System
2.1. Wound Healing Process
2.1.1. Hemostasis
2.1.2. Inflammation
2.1.3. Proliferation
2.1.4. Remodeling
2.2. Ideal Wound Care System
3. Electrospun Nanofibers for Wound Dressing
3.1. Electrospinning Technology
3.2. Type and Structure of Electrospun Nanofibers
4. Electrospun PAN Nanofiber
4.1. Natural Derivatives and Synthetic Additives of PAN Electrospun Fiber Systems for Wound Dressings

4.2. Electrospun Fibers Blended with PAN and Other Polymers
5. Research Progress of PAN-Based Electrospun Fibers in the Development of Wound Dressings
5.1. Natural Derivatives and Synthetic Additives of PAN Electrospun Fiber Systems for Wound Dressings
5.1.1. Drug Load with Wound Healing Properties
5.1.2. Other Additives with Wound Healing Properties
5.2. Strategies to Compose Composite Structures from Monolithic Nanofibers in PAN Electrospun Fiber Systems for Wound Dressings
5.3. Wound Healing Effect of Different PAN-Based Nanofiber Wound Dressings
5.4. Development of Electrospinning Technology in the Application of Wound Dressing
6. Summary and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAN | polyacrylonitrile |
| C. albicans | Candida albicans |
| DMF | N,N-dimethylformamide |
| DMAc | N,N-dimethylacetamide |
| DMSO | dimethyl sulfoxide |
| TETA | triethylenetetramine |
| BSA | bovine serum albumin |
| bPEI | branched polyaniline |
| SA | sodium alginate |
| CS | chitosan |
| GEL | gelatin |
| HA | hyaluronic acid |
| PVA | polyvinyl alcohol |
| PAM | polyacrylamide |
| PEG | polyethylene glycol |
| PMMA | polymethylmethacrylate |
| PCL | polycaprolactone |
| PU | Polyurethane |
| PANI | polyaniline |
| E. coli | Escherichia coli |
| S. aureus | Staphylococcus aureus |
| DLF | diclofenac |
| MOF | Metal-organic framework |
| AgSD | silver sulfadiazine |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| NO | Nitric oxide |
| NOS | nitric oxide synthase |
| SNAP | S-nitroso-N-acetylpenicillamine |
| QAS | quaternary ammonium salt |
| STAC | QAS-octadecyltrimethylammonium chloride |
| DSs | diclofenac sodium salt |
| GENs | gentamicin sulfate |
| SFP | silk fibroin peptide |
| CIP | ciprofloxacin |
| PLA | polylactide |
| PSP | polystyrene pyridine |
| OXA | oxacillin |
| P2W18 | α-K6P2W18O62·14H2O |
| 1D | one-dimensional |
| 2D | two-dimensional |
| 3D | three-dimensional |
References
- Li, S.; Li, L.; Guo, C.; Qin, H.; Yu, X. A Promising Wound Dressing Material with Excellent Cytocompatibility and Proangiogenesis Action for Wound Healing: Strontium Loaded Silk Fibroin/Sodium Alginate (SF/SA) Blend Films. Int. J. Biol. Macromol. 2017, 104, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhao, X.; Li, M.; Yan, L.; Lu, Y.; Jiang, C.; Liu, Y.; Pan, Z.; Shi, J. Antibacterial and Wound Healing–Promoting Effect of Sponge-Like Chitosan-Loaded Silver Nanoparticles Biosynthesized by Iturin. Int. J. Biol. Macromol. 2021, 181, 1183–1195. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, Z.; Abubaker, M.A.; Ding, L.; Zhang, J.; Yang, S.; Fan, Z. Antibacterial Polyvinyl Alcohol/Bacterial Cellulose/Nano-Silver Hydrogels That Effectively Promote Wound Healing. Mater. Sci. Eng. C 2021, 126, 112171. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, X.; Liu, P.; Zhang, M.; Wang, C.; Zhang, B. Multifunctional Chitosan/Polycaprolactone Nanofiber Scaffolds with Varied Dual-Drug Release for Wound-Healing Applications. ACS Biomater. Sci. Eng. 2020, 6, 4666–4676. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ma, B.C.; Reinholz, J.; Li, Q.; Wang, J.; Zhang, K.A.I.; Landfester, K.; Crespy, D. Efficient Nanofibrous Membranes for Antibacterial Wound Dressing and UV Protection. ACS Appl. Mater. Interfaces 2016, 8, 29915–29922. [Google Scholar] [CrossRef]
- Liang, D.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. Novel Asymmetric Wettable AgNPs/Chitosan Wound Dressing: In Vitro and in Vivo Evaluation. ACS Appl. Mater. Interfaces 2016, 8, 3958–3968. [Google Scholar] [CrossRef]
- Lan, G.; Li, Q.; Lu, F.; Yu, K.; Lu, B.; Bao, R.; Dai, F. Improvement of Platelet Aggregation and Rapid Induction of Hemostasis in Chitosan Dressing Using Silver Nanoparticles. Cellulose 2020, 27, 385–400. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, L.; Hong, F.F. A Biodegradable Antibacterial Nanocomposite Based on Oxidized Bacterial Nanocellulose for Rapid Hemostasis and Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 3382–3392. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, F.; Hua, Y.; Zhang, X.; Ni, C.; Pan, D.; Zhang, Y.; Jiang, D.; Yang, L.; Lin, Q. A Strongly Adhesive Hemostatic Hydrogel for the Repair of Arterial and Heart Bleeds. Nat. Commun. 2019, 10, 2060. [Google Scholar] [CrossRef]
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic Materials for Wound Healing Applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef]
- Morgado, P.I.; Aguiar-Ricardo, A.; Correia, I.J. Asymmetric Membranes as Ideal Wound Dressings: An Overview on Production Methods, Structure, Properties and Performance Relationship. J. Membr. Sci. 2015, 490, 139–151. [Google Scholar] [CrossRef]
- Patil, P.P.; Reagan, M.R.; Bohara, R.A. Silk Fibroin and Silk-Based Biomaterial Derivatives for Ideal Wound Dressings. Int. J. Biol. Macromol. 2020, 164, 4613–4627. [Google Scholar] [CrossRef] [PubMed]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent Advances on Antimicrobial Wound Dressing: A Review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahin, H.; Aldo, R.B. Antibacterial Biohybrid Nanofibers for Wound Dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef]
- Guebitz, G.M.; Nyanhongo, G.S. Enzymes as Green Catalysts and Interactive Biomolecules in Wound Dressing Hydrogels. Trends Biotechnol. 2018, 36, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, G.; Yu, Y.; Zhang, Y.; Li, X.; Wang, S. Design of Biocompatible Chitosan/Polyaniline/Laponite Hydrogel with Photothermal Conversion Capability. Biomolecules 2022, 12, 1089. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/Stimuli-Responsive Hydrogels: Cutting-Edge Platforms for Tissue Engineering and Other Biomedical Applications. Mater. Today Bio. 2022, 13, 100186. [Google Scholar] [CrossRef]
- Thanka Rajan, S.; Subramanian, B.; Arockiarajan, A. A Comprehensive Review on Biocompatible Thin Films for Biomedical Application. Ceram. Int. 2022, 48, 4377–4400. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial Biomaterials for Skin Wound Dressing. Asian J. Pharm. Sci. 2022, 17, 353–384. [Google Scholar] [CrossRef] [PubMed]
- Aycan, D.; Selmi, B.; Kelel, E.; Yildirim, T.; Alemdar, N. Conductive Polymeric Film Loaded with Ibuprofen as a Wound Dressing Material. Eur. Polym. J. 2019, 121, 109308. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Bi, S.; Wang, Y.; Chen, X.; Qiu, L.; Sun, J. Optically Transparent Antibacterial Films Capable of Healing Multiple Scratches. Adv. Funct. Mater. 2014, 24, 403–411. [Google Scholar] [CrossRef]
- Ngece, K.; Aderibigbe, B.A.; Ndinteh, D.T.; Fonkui, Y.T.; Kumar, P. Alginate-Gum Acacia Based Sponges as Potential Wound Dressings for Exuding and Bleeding Wounds. Int. J. Biol. Macromol. 2021, 172, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, X.; Lou, T. Simultaneous Adsorption for Cationic and Anionic Dyes Using Chitosan/Electrospun Sodium Alginate Nanofiber Composite Sponges. Carbohydr. Polym. 2022, 276, 118728. [Google Scholar] [CrossRef]
- Liu, C.; Liu, C.; Yu, S.; Wang, N.; Yao, W.; Liu, X.; Sun, G.; Song, Q.; Qiao, W. Efficient Antibacterial Dextran-Montmorillonite Composite Sponge for Rapid Hemostasis with Wound Healing. Int. J. Biol. Macromol. 2020, 160, 1130–1143. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Zayed, M.A.; El-dek, S.I.; Hady, M.A.; El Sherbiny, D.H.; Uskoković, V. Nanofibrous ε-Polycaprolactone Scaffolds Containing Ag-Doped Magnetite Nanoparticles: Physicochemical Characterization and Biological Testing for Wound Dressing Applications in Vitro and in Vivo. Bioact. Mater. 2021, 6, 2070–2088. [Google Scholar] [CrossRef]
- Asghari, F.; Rabiei Faradonbeh, D.; Malekshahi, Z.V.; Nekounam, H.; Ghaemi, B.; Yousefpoor, Y.; Ghanbari, H.; Faridi-Majidi, R. Hybrid PCL/Chitosan-PEO Nanofibrous Scaffolds Incorporated with A. Euchroma Extract for Skin Tissue Engineering Application. Carbohydr. Polym. 2022, 278, 118926. [Google Scholar] [CrossRef]
- Cheng, R.; Liu, L.; Xiang, Y.; Lu, Y.; Deng, L.; Zhang, H.; Santos, H.A.; Cui, W. Advanced Liposome-Loaded Scaffolds for Therapeutic and Tissue Engineering Applications. Biomaterials 2020, 232, 119706. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Huang, J.; Fang, Y.; Huang, H.; Wu, J. 1D, 2D, and 3D Scaffolds Promoting Angiogenesis for Enhanced Wound Healing. Chem. Eng. J. 2022, 437, 134690. [Google Scholar] [CrossRef]
- Nemati, S.; Kim, S.; Shin, Y.M.; Shin, H. Current Progress in Application of Polymeric Nanofibers to Tissue Engineering. Nano Converg. 2019, 6, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Li, L.; Yang, D.; Nie, J.; Ma, G. Electrospun Core–Shell Fibrous 2D Scaffold with Biocompatible Poly(Glycerol Sebacate) and Poly-l-Lactic Acid for Wound Healing. Adv. Fiber Mater. 2020, 2, 105–117. [Google Scholar] [CrossRef] [Green Version]
- El-Shanshory, A.A.; Agwa, M.M.; Abd-Elhamid, A.I.; Soliman, H.M.A.; Mo, X.; Kenawy, E.-R. Metronidazole Topically Immobilized Electrospun Nanofibrous Scaffold: Novel Secondary Intention Wound Healing Accelerator. Polymers 2022, 14, 454. [Google Scholar] [CrossRef]
- Yao, Z.-Y.; Qin, J.; Gong, J.-S.; Ye, Y.-H.; Qian, J.-Y.; Li, H.; Xu, Z.-H.; Shi, J.-S. Versatile Strategies for Bioproduction of Hyaluronic Acid Driven by Synthetic Biology. Carbohydr. Polym. 2021, 264, 118015. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Sheng, D.; Jiang, L.; Shafiq, M.; Khan, A.U.R.; Hashim, R.; Chen, Y.; Li, B.; Xie, X.; Chen, J.; et al. Vascular Endothelial Growth Factor-Capturing Aligned Electrospun Polycaprolactone/Gelatin Nanofibers Promote Patellar Ligament Regeneration. Acta Biomater. 2022, 140, 233–246. [Google Scholar] [CrossRef]
- Song, Y.; Huang, H.; He, D.; Yang, M.; Wang, H.; Zhang, H.; Li, J.; Li, Y.; Wang, C. Gallic Acid/2-Hydroxypropyl-β-Cyclodextrin Inclusion Complexes Electrospun Nanofibrous Webs: Fast Dissolution, Improved Aqueous Solubility and Antioxidant Property of Gallic Acid. Chem. Res. Chin. Univ. 2021, 37, 450–455. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.-G.; Wang, L.; Li, X.; Williams, G.R. Energy-Saving Electrospinning with a Concentric Teflon-Core Rod Spinneret to Create Medicated Nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef]
- Brimo, N.; Serdaroğlu, D.Ç.; Uysal, B. Comparing Antibiotic Pastes with Electrospun Nanofibers as Modern Drug Delivery Systems for Regenerative Endodontics. Curr. Drug Deliv. 2022, 19, 1–14. [Google Scholar] [CrossRef]
- Song, X.; Jiang, Y.; Zhang, W.; Elfawal, G.; Wang, K.; Jiang, D.; Hong, H.; Wu, J.; He, C.; Mo, X. Transcutaneous Tumor Vaccination Combined with Anti-Programmed Death-1 Monoclonal Antibody Treatment Produces a Synergistic Antitumor Effect. Acta Biomater. 2022, 140, 247–260. [Google Scholar] [CrossRef]
- Yu, D.G.; Lv, H. Preface-striding into nano drug delivery. Curr. Drug Deliv. 2022, 19, 1–3. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.-G.; Kim, I. Recent Advances in Poly(α-L-glutamic acid)-Based Nanomaterials for Drug Delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tan, Y.; Li, D.; Xu, G.; Yin, D.; Xiao, Y.; Xu, T.; Chen, X.; Zhu, X.; Shi, X. Negative Isolation of Circulating Tumor Cells Using a Microfluidic Platform Integrated with Streptavidin-Functionalized PLGA Nanofibers. Adv. Fiber Mater. 2021, 3, 192–202. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.-G.; Wang, K. Electrospun Nanofiber Membranes for Air Filtration: A Review. Nanomaterials 2022, 12, 1077. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, M.; Wang, P.; Xu, X.; Liu, Y.; Yu, D.-G. Ingenious Construction of Ni(DMG)2/TiO2-Decorated Porous Nanofibers for the Highly Efficient Photodegradation of Pollutants in Water. Colloids Surf. A 2022, 650, 129561. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Galhoum, A.A.; Alshahrie, A.; Al-Turki, Y.A.; Al-Amri, A.M.; Wageh, S. Mesoporous Magnetic Cysteine Functionalized Chitosan Nanocomposite for Selective Uranyl Ions Sorption: Experimental, Structural Characterization, and Mechanistic Studies. Polymers 2022, 14, 2568. [Google Scholar] [CrossRef]
- Xu, X.; Lv, H.; Zhang, M.; Wang, M.; Zhou, Y.; Liu, Y.; Yu, D.-G. Progress on electrospun nanofiber for heavy metal wastewater applications: A review. Front. Chem. Sci. Eng. 2022, 16, 1–17. [Google Scholar] [CrossRef]
- Xie, L.; Li, Z.; Li, X.; Wenlong, W.; Hanjiang, Y. Electrospun Copper Oxide Nanofibers and Catalysis for Combustion of Ammonium Perchlorate. Ferroelectrics 2019, 549, 23–28. [Google Scholar] [CrossRef]
- Wang, L.; Haugen, N.O.; Wu, Z.; Shu, X.; Jia, Y.; Ma, J.; Yu, S.; Li, H.; Chai, Q. Ferroelectric BaTiO3@ZnO Heterostructure Nanofibers with Enhanced Pyroelectrically-Driven-Catalysis. Ceram. Int. 2019, 45, 90–95. [Google Scholar] [CrossRef]
- Wu, H.; Lin, D.; Pan, W. Fabrication, Assembly, and Electrical Characterization of CuO Nanofibers. Appl. Phys. Lett. 2006, 89, 133125. [Google Scholar] [CrossRef]
- Yoo, T.-H.; Hwang, D.K. Field Effect Transistors Based on One-Dimensional, Metal-Oxide Semiconducting Nanofiber Mats. J. Korean Phys. Soc. 2019, 74, 827–830. [Google Scholar] [CrossRef]
- Lee, M.Y.; Hong, J.; Lee, E.K.; Yu, H.; Kim, H.; Lee, J.U.; Lee, W.; Oh, J.H. Highly Flexible Organic Nanofiber Phototransistors Fabricated on a Textile Composite for Wearable Photosensors. Adv. Funct. Mater. 2016, 26, 1445–1453. [Google Scholar] [CrossRef]
- Chou, C.-C.; Wu, H.-C.; Lin, C.-J.; Ghelichkhani, E.; Chen, W.-C. Morphology and Field-Effect Transistor Characteristics of Electrospun Nanofibers Prepared From Crystalline Poly(3-Hexylthiophene) and Polyacrylate Blends. Macromol. Chem. Phys. 2013, 214, 751–760. [Google Scholar] [CrossRef]
- Danilo, M.S.; Correa, D.S.; Medeiros, E.S.; Oliveira, J.E.; Mattoso, L.H.C. Functional Polymer Nanofibers: From Spinning Fabrication Techniques to Recent Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 45673–45701. [Google Scholar] [CrossRef]
- Feng, G.; Zhou, H.; Zhou, G.; Yang, H.; Deng, G.; Zhou, S. Optical Properties of a Nanofiber with Metal Cladding. J. Korean Phys. Soc. 2011, 58, 930–933. [Google Scholar] [CrossRef]
- Kim, T.; Naoki, W.; Miyawaki, J.; Park, J.-I.; Lee, C.; Jung, H.-K.; Jeon, M.-S.; Kim, H.-J.; Yoon, S.-H. Synthesis of Surface-Replicated Ultra-Thin Silica Hollow Nanofibers Using Structurally Different Carbon Nanofibers as Templates. J. Solid State Chem. 2019, 272, 21–26. [Google Scholar] [CrossRef]
- Augustine, G.; Aarthy, M.; Thiagarajan, H.; Selvaraj, S.; Kamini, N.R.; Shanmugam, G.; Ayyadurai, N. Self-Assembly and Mechanical Properties of Engineered Protein Based Multifunctional Nanofiber for Accelerated Wound Healing. Adv. Healthc. Mater. 2021, 10, 2001832. [Google Scholar] [CrossRef]
- Janghela, S.; Devi, S.; Kambo, N.; Roy, D.; Mukhopadhyay, K.; Prasad, N.E. Microphase Separation in Oriented Polymeric Chains at the Surface of Nanomaterials during Nanofiber Formation. Soft Matter 2019, 15, 6811–6818. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of Electrospinning Technique for Biomedical Applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef] [Green Version]
- Hooshmand, S.; Aitomäki, Y.; Norberg, N.; Mathew, A.P.; Oksman, K. Dry-Spun Single-Filament Fibers Comprising Solely Cellulose Nanofibers from Bioresidue. ACS Appl. Mater. Interfaces 2015, 7, 13022–13028. [Google Scholar] [CrossRef]
- Wang, L.; Ezazi, N.Z.; Liu, L.; Ajdary, R.; Xiang, W.; Borghei, M.; Santos, H.A.; Rojas, O.J. Microfibers Synthesized by Wet-Spinning of Chitin Nanomaterials: Mechanical, Structural and Cell Proliferation Properties. RSC Adv. 2020, 10, 29450–29459. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, N.; Cui, W.; Zhang, P.; Chen, X. A Novel Nano/Micro-Fibrous Scaffold by Melt-Spinning Method for Bone Tissue Engineering. J. Bionic Eng. 2015, 12, 117–128. [Google Scholar] [CrossRef]
- Gonzaga, L.A.C.; Martins, M.C.F.; Correa, A.C.; Facchinatto, W.M.; Silva, C.M.P.; Colnago, L.A.; Mattoso, L.H.C. Production of Carbon Nanofibers from PAN and Lignin by Solution Blow Spinning. J. Polym. Res. 2021, 28, 237. [Google Scholar] [CrossRef]
- Khang, A.; Ravishankar, P.; Krishnaswamy, A.; Anderson, P.K.; Cone, S.G.; Liu, Z.; Qian, X.; Balachandran, K. Engineering Anisotropic Biphasic Janus-Type Polymer Nanofiber Scaffold Networks via Centrifugal Jet Spinning. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2455–2464. [Google Scholar] [CrossRef]
- Cui, T.; Yu, J.; Li, Q.; Wang, C.-F.; Chen, S.; Li, W.; Wang, G. Large-Scale Fabrication of Robust Artificial Skins from a Biodegradable Sealant-Loaded Nanofiber Scaffold to Skin Tissue via Microfluidic Blow-Spinning. Adv. Mater. 2020, 32, 2000982. [Google Scholar] [CrossRef] [PubMed]
- Gruppuso, M.; Turco, G.; Marsich, E.; Porrelli, D. Polymeric Wound Dressings, an Insight into Polysaccharide-Based Electrospun Membranes. Appl. Mater. Today 2021, 24, 101148. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Jiang, S.; Schmalz, H.; Agarwal, S.; Greiner, A. Electrospinning of ABS Nanofibers and Their High Filtration Performance. Adv. Fiber Mater. 2020, 2, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.; Gong, X.; Jiao, W.; Li, Y.; Yin, X.; Si, Y.; Yu, J.; Ding, B. In-Situ Electrospinning of Thymol-Loaded Polyurethane Fibrous Membranes for Waterproof, Breathable, and Antibacterial Wound Dressing Application. J. Colloid Interf. Sci. 2021, 592, 310–318. [Google Scholar] [CrossRef]
- Séon-Lutz, M.; Couffin, A.-C.; Vignoud, S.; Schlatter, G.; Hébraud, A. Electrospinning in Water and in Situ Crosslinking of Hyaluronic Acid/Cyclodextrin Nanofibers: Towards Wound Dressing with Controlled Drug Release. Carbohydr. Polym. 2019, 207, 276–287. [Google Scholar] [CrossRef]
- Gul, A.; Gallus, I.; Tegginamath, A.; Maryska, J.; Yalcinkaya, F. Electrospun Antibacterial Nanomaterials for Wound Dressings Applications. Membranes 2021, 11, 908. [Google Scholar] [CrossRef]
- Yeniay, E.; Öcal, L.; Altun, E.; Giray, B.; Nuzhet Oktar, F.; Talat Inan, A.; Ekren, N.; Kilic, O.; Gunduz, O. Nanofibrous Wound Dressing Material by Electrospinning Method. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 11–18. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in Nanofibers for Wound Dressing: A Review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Liu, G.-S.; Yan, X.; Yan, F.-F.; Chen, F.-X.; Hao, L.-Y.; Chen, S.-J.; Lou, T.; Ning, X.; Long, Y.-Z. In Situ Electrospinning Iodine-Based Fibrous Meshes for Antibacterial Wound Dressing. Nanoscale Res. Lett. 2018, 13, 309. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A Review on Electrospinning Design and Nanofibre Assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.-Y.; Jing, X.; Huang, H.-X.; Turng, L.-S. Instantaneous Self-Assembly of Three-Dimensional Silica Fibers in Electrospinning: Insights into Fiber Deposition Behavior. Mater. Lett. 2017, 204, 45–48. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, L.; Wang, X.; Shao, Z.; Kong, B. Electrospinning Super-Assembly of Ultrathin Fibers from Single- to Multi-Taylor Cone Sites. Appl. Mater. Today 2022, 26, 101272. [Google Scholar] [CrossRef]
- Xue, Y.; Ravishankar, P.; Zeballos, M.A.; Sant, V.; Balachandran, K.; Sant, S. Valve Leaflet-Inspired Elastomeric Scaffolds with Tunable and Anisotropic Mechanical Properties. Polym. Adv. Technol. 2020, 31, 94–106. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Lee, P.-Y.; Tuan-Mu, H.-Y.; Li, C.-Y.; Hu, J.-J. Fabrication of a Mechanically Anisotropic Poly(Glycerol Sebacate) Membrane for Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 760–770. [Google Scholar] [CrossRef]
- Anyaegbu, C.E.; Zhang, H.; Xiao, J.; Tao, M.; Ma, N.; Zhang, W. Tertiary Amine-Bisquaternary Ammonium Functionalized Polyacrylonitrile Fiber for Catalytic Synthesis of Pyran-Annulated Heterocycles. React. Funct. Polym. 2022, 172, 105201. [Google Scholar] [CrossRef]
- Li, Y.; Abedalwafa, M.A.; Ni, C.; Sanbhal, N.; Wang, L. Removal and Direct Visual Monitoring of Lead(II) Using Amino Acids Functionalized Polyacrylonitrile Nanofibrous Membranes. React. Funct. Polym. 2019, 138, 18–28. [Google Scholar] [CrossRef]
- Wang, F.; Song, Y.; Liang, S.; Yu, Y.; Liang, J.; Jiang, M. Polyamidoxime Nanoparticles/Polyvinyl Alcohol Composite Chelating Nanofibers Prepared by Centrifugal Spinning for Uranium Extraction. React. Funct. Polym. 2021, 159, 104812. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, M.; Lv, H.; Zhou, Y.; Yang, Y.; Yu, D.-G. Electrospun Polyacrylonitrile-Based Lace Nanostructures and Their Cu(II) Adsorption. Sep. Purif. Technol. 2022, 288, 120643. [Google Scholar] [CrossRef]
- Patil, N.; Oh, J.H.; Khatri, S.; Saed, M.A.; Naraghi, M.; Green, M.J. Radio Frequency Heating Response of Polyacrylonitrile (PAN) Films and Nanofiber Mats. ACS Appl. Polym. Mater. 2021, 3, 3125–3130. [Google Scholar] [CrossRef]
- Xu, G.; Cao, J.; Zhao, Y.; Zheng, L.; Tao, M.; Zhang, W. Phosphorylated Polyacrylonitrile Fibers as an Efficient and Greener Acetalization Catalyst. Chem. Asian J. 2017, 12, 2565–2575. [Google Scholar] [CrossRef]
- Kim, S.E.; Tiwari, A.P. Mussel-Inspired Polydopamine-Enabled in Situ-Synthesized Silver Nanoparticle-Anchored Porous Polyacrylonitrile Nanofibers for Wound-Healing Applications. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 471–480. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Hurlow, J.; Bowler, P.G. Acute and Chronic Wound Infections: Microbiological, Immunological, Clinical and Therapeutic Distinctions. J. Wound Care 2022, 31, 436–445. [Google Scholar] [CrossRef]
- Ding, X.; Tang, Q.; Xu, Z.; Xu, Y.; Zhang, H.; Zheng, D.; Wang, S.; Tan, Q.; Maitz, J.; Maitz, P.K. Challenges and Innovations in Treating Chronic and Acute Wound Infections: From Basic Science to Clinical Practice. Burns Trauma 2022, 10, tkac014. [Google Scholar] [CrossRef]
- Juncos Bombin, A.D.; Dunne, N.J.; McCarthy, H.O. Electrospinning of Natural Polymers for the Production of Nanofibres for Wound Healing Applications. Mater. Sci. Eng. C 2020, 114, 110994. [Google Scholar] [CrossRef]
- Zhang, B.; Pang, Z.; Hu, Y. Targeting Hemostasis-Related Moieties for Tumor Treatment. Thromb. Res. 2020, 187, 186–196. [Google Scholar] [CrossRef]
- Souza, G.S.; Jesus Sonego, L.; Santos Mundim, A.C.; Miranda Moraes, J.; Sales-Campos, H.; Lorenzón, E.N. Antimicrobial-Wound Healing Peptides: Dual-Function Molecules for the Treatment of Skin Injuries. Peptides 2022, 148, 170707. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J. Healing Mechanisms in Cutaneous Wounds: Tipping the Balance. Tissue Eng. Part B Rev. 2022. [Google Scholar] [CrossRef] [PubMed]
- Opneja, A.; Kapoor, S.; Stavrou, E.X. Contribution of Platelets, the Coagulation and Fibrinolytic Systems to Cutaneous Wound Healing. Thromb. Res. 2019, 179, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Yazarlu, O.; Iranshahi, M.; Kashani, H.R.K.; Reshadat, S.; Habtemariam, S.; Iranshahy, M.; Hasanpour, M. Perspective on the Application of Medicinal Plants and Natural Products in Wound Healing: A Mechanistic Review. Pharmacol. Res. 2021, 174, 105841. [Google Scholar] [CrossRef]
- Wang, M.-L.; Huang, X.W.; Zheng, H.X.; Tang, Y.M.; Zeng, K.; Shao, L.Q.; Li, L. Nanomaterials Applied in Wound Healing: Mechanisms, Limitations and Perspectives. J. Control. Rel. 2021, 337, 236–247. [Google Scholar] [CrossRef]
- Chen, S.; Lu, J.; You, T.; Sun, D. Metal-Organic Frameworks for Improving Wound Healing. Coord. Chem. Rev. 2021, 439, 213929. [Google Scholar] [CrossRef]
- Xiang, J.; Shen, L.; Hong, Y. Status and Future Scope of Hydrogels in Wound Healing: Synthesis, Materials and Evaluation. Eur. Polym. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
- Wang, P.-H.; Huang, B.-S.; Horng, H.-C.; Yeh, C.-C.; Chen, Y.-J. Wound Healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, A.; Dey, A.D.; Behl, T.; Chadha, S. Stem Cells and Growth Factors-Based Delivery Approaches for Chronic Wound Repair and Regeneration: A Promise to Heal from Within. Life Sci. 2021, 268, 118932. [Google Scholar] [CrossRef]
- Li, J.; Guan, S.; Su, J.; Liang, J.; Cui, L.; Zhang, K. The Development of Hyaluronic Acids Used for Skin Tissue Regeneration. Curr. Drug Deliv. 2021, 18, 836–846. [Google Scholar] [CrossRef]
- Feng, X.; Hao, J. Identifying New Pathways and Targets for Wound Healing and Therapeutics from Natural Sources. Curr. Drug Deliv. 2021, 18, 1064–1084. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H. Drug Delivery Systems and Materials for Wound Healing Applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Whittam, A.J.; Maan, Z.N.; Duscher, D.; Wong, V.W.; Barrera, J.A.; Januszyk, M.; Gurtner, G.C. Challenges and Opportunities in Drug Delivery for Wound Healing. Adv. Wound Care 2016, 5, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Helmiyati; Aprilliza, M. Characterization and Properties of Sodium Alginate from Brown Algae Used as an Ecofriendly Superabsorbent. IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 012019. [Google Scholar] [CrossRef]
- Kim, J.O.; Park, J.K.; Kim, J.H.; Jin, S.G.; Yong, C.S.; Li, D.X.; Choi, J.Y.; Woo, J.S.; Yoo, B.K.; Lyoo, W.S. Development of Polyvinyl Alcohol–Sodium Alginate Gel-Matrix-Based Wound Dressing System Containing Nitrofurazone. Int. J. Pharm. 2008, 359, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L. Polysaccharides Composite Materials for Rapid Hemostasis. J. Drug Deliv. Sci. Technol. 2021, 66, 102890. [Google Scholar] [CrossRef]
- Choudhary, M.; Chhabra, P.; Tyagi, A.; Singh, H. Scar Free Healing of Full Thickness Diabetic Wounds: A Unique Combination of Silver Nanoparticles as Antimicrobial Agent, Calcium Alginate Nanoparticles as Hemostatic Agent, Fresh Blood as Nutrient/Growth Factor Supplier and Chitosan as Base Matrix. Int. J. Biol. Macromol. 2021, 178, 41–52. [Google Scholar] [CrossRef]
- Cesur, S.; Oktar, F.N.; Ekren, N.; Kilic, O.; Alkaya, D.B.; Seyhan, S.A.; Ege, Z.R.; Lin, C.-C.; Kuruca, S.E.; Erdemir, G. Preparation and Characterization of Electrospun Polylactic Acid/Sodium Alginate/Orange Oyster Shell Composite Nanofiber for Biomedical Application. J. Aust. Ceram. Soc. 2020, 56, 533–543. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, P.; Yang, Y.; Yu, D.-G. Electrospun Beads-on-the-String Nanoproducts: Preparation and Drug Delivery Application. Curr. Drug Deliv. 2022, 19, 1–13. [Google Scholar] [CrossRef]
- Nischwitz, S.P.; Hofmann, E.; Kamolz, L.-P. The Ideal Wound Dressing—Beyond the Ideal: A Short Comment on ‘Properties of an Ideal Burn Dressing: A Survey of Burn Survivors and Front-Line Burn Healthcare Providers’ by T. Carta, J.P. Gawaziuk et al. Burns 2019, 45, 1485–1486. [Google Scholar] [CrossRef] [PubMed]
- Selig, H.F.; Lumenta, D.B.; Giretzlehner, M.; Jeschke, M.G.; Upton, D.; Kamolz, L.P. The Properties of an “Ideal” Burn Wound Dressing—What Do We Need in Daily Clinical Practice? Results of a Worldwide Online Survey among Burn Care Specialists. Burns 2012, 38, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Jiang, W.; Shen, L.; Zhang, G.; Gao, Y.; Yang, Y.; Yu, D.-G. Electrospun Hybrid Films for Fast and Convenient Delivery of Active Herb Extracts. Membranes 2022, 12, 398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, S.; Qin, Y.; Li, C. Functional Electrospun Nanocomposites for Efficient Oxygen Reduction Reaction. Chem. Res. Chin. Univ. 2021, 37, 379–393. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Cheng, H.; Li, G.; Cho, H.; Jiang, M.; Gao, Q.; Zhang, X. Developments of Advanced Electrospinning Techniques: A Critical Review. Adv. Mater. Technol. 2021, 6, 2100410. [Google Scholar] [CrossRef]
- Liu, M.; Duan, X.-P.; Li, Y.-M.; Yang, D.-P.; Long, Y.-Z. Electrospun Nanofibers for Wound Healing. Mater. Sci. Eng. C 2017, 76, 1413–1423. [Google Scholar] [CrossRef]
- Chang, S.; Wang, M.; Zhang, F.; Liu, Y.; Liu, X.; Yu, D.G.; Shen, H. Sheath-Separate-Core Nanocomposites Fabricated Using a Trifluid Electrospinning. Mater. Des. 2020, 192, 108782. [Google Scholar] [CrossRef]
- He, H.; Wu, M.; Zhu, J.; Yang, Y.; Ge, R.; Yu, D.-G. Engineered Spindles of Little Molecules around Electrospun Nanofibers for Biphasic Drug Release. Adv. Fiber Mater. 2021, 4, 305–317. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Xu, Y.; Shi, X.; Zhang, M.; Huang, Y.; Liang, Y.; Chen, Y.; Ji, W.; Kim, J.R. Engineering of Hollow Polymeric Nanosphere-Supported Imidazolium-Based Ionic Liquids with Enhanced Antimicrobial Activities. Nano Res. 2022, 15, 5556–5568. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, X.; Liu, P.; Yu, D.G.; Ge, R. Electrospun Nanofibers-Based Glucose Sensors for Glucose Detection. Front. Chem. 2022, 10, 944428. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Zeng, L.; Qiao, Z.; Liu, X.; Liu, H.; Zhang, J.; Ding, J. Fabrication of Electrospun Polymer Nanofibers with Diverse Morphologies. Molecules 2019, 24, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivan, M.; Madheswaran, D.; Valtera, J.; Kostakova, E.K.; Lukas, D. Alternating Current Electrospinning: The Impacts of Various High-Voltage Signal Shapes and Frequencies on the Spinnability and Productivity of Polycaprolactone Nanofibers. Mater. Des. 2022, 213, 110308. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Zhou, W.; Yu, D. Electrospun Medical Sutures for Wound Healing: A Review. Polymers 2022, 14, 1637. [Google Scholar] [CrossRef]
- Zhao, K.; Lu, Z.-H.; Zhao, P.; Kang, S.-X.; Yang, Y.-Y.; Yu, D.-G. Modified Tri–Axial Electrospun Functional Core–Shell Nanofibrous Membranes for Natural Photodegradation of Antibiotics. Chem. Eng. J. 2021, 425, 131455. [Google Scholar] [CrossRef]
- Helgeson, M.E.; Wagner, N.J. A Correlation for the Diameter of Electrospun Polymer Nanofibers. AIChE J. 2007, 53, 51–55. [Google Scholar] [CrossRef]
- Erencia, M.; Cano, F.; Tornero, J.A.; Macanás, J.; Carrillo, F. Resolving the Electrospinnability Zones and Diameter Prediction for the Electrospinning of the Gelatin/Water/Acetic Acid System. Langmuir 2014, 30, 7198–7205. [Google Scholar] [CrossRef]
- Wu, T.; Ding, M.; Shi, C.; Qiao, Y.; Wang, P.; Qiao, R.; Wang, X.; Zhong, J. Resorbable Polymer Electrospun Nanofibers: History, Shapes and Application for Tissue Engineering. Chin. Chem. Lett. 2020, 31, 617–625. [Google Scholar] [CrossRef]
- Ali, S.; Khatri, Z.; Oh, K.W.; Kim, I.-S.; Kim, S.H. Zein/Cellulose Acetate Hybrid Nanofibers: Electrospinning and Characterization. Macromol. Res. 2014, 22, 971–977. [Google Scholar] [CrossRef]
- Shin, J.; Lee, S. Encapsulation of Phytoncide in Nanofibers by Emulsion Electrospinning and Their Antimicrobial Assessment. Fibers Polym. 2018, 19, 627–634. [Google Scholar] [CrossRef]
- Koplányi, G.; Sánta-Bell, E.; Molnár, Z.; Tóth, G.D.; Józó, M.; Szilágyi, A.; Ender, F.; Pukánszky, B.; Vértessy, B.G.; Poppe, L. Entrapment of Phenylalanine Ammonia-Lyase in Nanofibrous Polylactic Acid Matrices by Emulsion Electrospinning. Catalysts 2021, 11, 1149. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Gao, Y.; Yu, D.-G.; Liu, P. Elaborate Design of Shell Component for Manipulating the Sustained Release Behavior from Core–Shell Nanofibres. J. Nanobiotechnol. 2022, 20, 244. [Google Scholar] [CrossRef] [PubMed]
- Bukhary, H.; Williams, G.R.; Orlu, M. Fabrication of Electrospun Levodopa-Carbidopa Fixed-Dose Combinations. Adv. Fiber Mater. 2020, 2, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Dong, J.; Zhang, Y.; Chai, S.; Wang, X.; Kang, S.; Yu, D.; Wang, P.; Jiang, Q. Gold Nanoparticles-Loaded Polyvinylpyrrolidone/Ethylcellulose Coaxial Electrospun Nanofibers with Enhanced Osteogenic Capability for Bone Tissue Regeneration. Mater. Des. 2021, 212, 110240. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Liu, Y.; Gao, Y.; Liu, P. Electrospun Coaxial Fibers to Optimize the Release of Poorly Water-Soluble Drug. Polymers 2022, 14, 469. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, D.-G.; Williams, G.R.; Bligh, S.W.A. Co-Loading of Inorganic Nanoparticles and Natural Oil in the Electrospun Janus Nanofibers for a Synergetic Antibacterial Effect. Pharmaceutics 2022, 14, 1208. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Lu, X.; Murugadoss, V.; Huang, M.; Yang, H.; Wan, F.; Yu, D.-G.; Guo, Z. Electrospun Structural Nanohybrids Combining Three Composites for Fast Helicide Delivery. Adv. Compos. Hybrid Mater. 2022, 5, 1017–1029. [Google Scholar] [CrossRef]
- Wang, M.; Hou, J.; Yu, D.-G.; Li, S.; Zhu, J.; Chen, Z. Electrospun Tri-Layer Nanodepots for Sustained Release of Acyclovir. J. Alloys Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Ding, Y.; Dou, C.; Chang, S.; Xie, Z.; Yu, D.-G.; Liu, Y.; Shao, J. Core–Shell Eudragit S100 Nanofibers Prepared via Triaxial Electrospinning to Provide a Colon-Targeted Extended Drug Release. Polymers 2020, 12, 2034. [Google Scholar] [CrossRef]
- Zhan, L.; Deng, J.; Ke, Q.; Li, X.; Ouyang, Y.; Huang, C.; Liu, X.; Qian, Y. Grooved Fibers: Preparation Principles through Electrospinning and Potential Applications. Adv. Fiber Mater. 2022, 4, 203–213. [Google Scholar] [CrossRef]
- Liu, R.; Hou, L.; Yue, G.; Li, H.; Zhang, J.; Liu, J.; Miao, B.; Wang, N.; Bai, J.; Cui, Z. Progress of Fabrication and Applications of Electrospun Hierarchically Porous Nanofibers. Adv. Fiber Mater. 2022, 4, 604–630. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Song, W.; Zhang, Y.; Yu, D.-G.; Liu, Y. Electrospun Core (HPMC–Acetaminophen)–Shell (PVP–Sucralose) Nanohybrids for Rapid Drug Delivery. Gels 2022, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, L. Preparation and Characterization of Porous Core-Shell Fibers for Slow Release of Tea Polyphenols. Polymers 2018, 10, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, S.Y.; Ren, J.; Vancso, G.J. Process Optimization and Empirical Modeling for Electrospun Polyacrylonitrile (PAN) Nanofiber Precursor of Carbon Nanofibers. Eur. Polym. J. 2005, 41, 2559–2568. [Google Scholar] [CrossRef]
- Isaac, B.; Taylor, R.M.; Reifsnider, K. Anisotropic Characterizations of Electrospun PAN Nanofiber Mats Using Design of Experiments. Nanomaterials 2020, 10, 2273. [Google Scholar] [CrossRef]
- Trabelsi, M.; Mamun, A.; Klöcker, M.; Brockhagen, B.; Kinzel, F.; Kapanadze, D.; Sabantina, L. Polyacrylonitrile (PAN) Nanofiber Mats for Mushroom Mycelium Growth Investigations and Formation of Mycelium-Reinforced Nanocomposites. J. Eng. Fiber. Fabr. 2021, 16, 155892502110379. [Google Scholar] [CrossRef]
- Sirelkhatim, N.; Parveen, A.; LaJeunesse, D.; Yu, D.; Zhang, L. Polyacrylonitrile Nanofibrous Mat from Electrospinning: Born with Potential Anti-Fungal Functionality. Eur. Polym. J. 2019, 119, 176–180. [Google Scholar] [CrossRef]
- Amiri, P.; Talebi, Z.; Semnani, D.; Bagheri, R.; Fashandi, H. Improved Performance of Bis-GMA Dental Composites Reinforced with Surface-Modified PAN Nanofibers. J. Mater. Sci. Mater. Med. 2021, 32, 82. [Google Scholar] [CrossRef]
- Gobi, N.; Priyanka, V.; Monnisha, G. Surface Modified Polyacrylonitrile/Polyamide Nanofibre Composite for Air Filtration. Polym. Polym. Compos. 2022, 30, 096739112210959. [Google Scholar] [CrossRef]
- Bode-Aluko, C.A.; Pereao, O.; Fatoba, O.; Petrik, L. Surface-Modified Polyacrylonitrile Nanofibres as Supports. Polym. Bull. 2017, 74, 2431–2442. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Q.; Yan, B.; Liu, B.; Gu, Y.; Lin, Y.; Shang, J.; Liu, W.; Chen, S.; Lan, J. Aminated Polyacrylonitrile Nanofiber Membranes for the Removal of Organic Dyes. ACS Appl. Nano Mater. 2022, 5, 1131–1140. [Google Scholar] [CrossRef]
- Huang, F.; Xu, Y.; Liao, S.; Yang, D.; Hsieh, Y.-L.; Wei, Q. Preparation of Amidoxime Polyacrylonitrile Chelating Nanofibers and Their Application for Adsorption of Metal Ions. Materials 2013, 6, 969–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Liu, Q.; Xia, Q.; Wang, Y. Facilely Cyclization-Modified PAN Nanofiber Substrate of Thin Film Composite Membrane for Ultrafast Polar Solvent Separation. J. Membr. Sci. 2022, 641, 119911. [Google Scholar] [CrossRef]
- Mei, Y.; Yao, C.; Fan, K.; Li, X. Surface Modification of Polyacrylonitrile Nanofibrous Membranes with Superior Antibacterial and Easy-Cleaning Properties through Hydrophilic Flexible Spacers. J. Membr. Sci. 2012, 417–418, 20–27. [Google Scholar] [CrossRef]
- Zhao, H.; He, W.; Wang, Y.; Zhang, X.; Li, Z.; Yan, S.; Zhou, W.; Wang, G. Biomineralization of Large Hydroxyapatite Particles Using Ovalbumin as Biosurfactant. Mater. Lett. 2008, 62, 3603–3605. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, Y.; Hao, H.; Zhang, Y.; Xu, X.; Tang, R. Therapeutic Potential of Biomineralization-Based Engineering. Adv. Ther. 2018, 1, 1800079. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Tsai, T.-Y.; Zarie, E.S.; Elbahri, M.; Young, T.-H.; Boccaccini, A.R. Bovine Serum Albumin (BSA)/Polyacrylonitrile (PAN) Biohybrid Nanofibers Coated with a Biomineralized Calcium Deficient Hydroxyapatite (HA) Shell for Wound Dressing. Mater. Sci. Eng. C 2020, 116, 111248. [Google Scholar] [CrossRef]
- Kang, Y.H.; Ahn, K.; Jeong, S.Y.; Bae, J.S.; Jin, J.S.; Kim, H.G.; Hong, S.W.; Cho, C.R. Effect of Plasma Treatment on Surface Chemical-Bonding States and Electrical Properties of Polyacrylonitrile Nanofibers. Thin Solid Film. 2011, 519, 7090–7094. [Google Scholar] [CrossRef]
- Zhang, W.; Yue, P.; Zhang, H.; Yang, N.; Li, C.; Li, J.H.; Meng, J.; Zhang, Q. Surface Modification of AO-PAN@OHec Nanofiber Membranes with Amino Acid for Antifouling and Hemocompatible Properties. Appl. Surf. Sci. 2019, 475, 934–941. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Shen, M.; Tan, X.; Ding, Y.; Jiang, Z.; Wang, C. Preparation of Phosphorylated Polyacrylonitrile-Based Nanofiber Mat and Its Application for Heavy Metal Ion Removal. Chem. Eng. J. 2015, 268, 290–299. [Google Scholar] [CrossRef]
- Zhao, R.; Li, Y.; Li, X.; Li, Y.; Sun, B.; Chao, S.; Wang, C. Facile Hydrothermal Synthesis of Branched Polyethylenimine Grafted Electrospun Polyacrylonitrile Fiber Membrane as a Highly Efficient and Reusable Bilirubin Adsorbent in Hemoperfusion. J. Colloid Interf. Sci. 2018, 514, 675–685. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-Based Composite Materials for Wound Dressing Application:A Mini Review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef] [PubMed]
- Del Gaudio, P.; Amante, C.; Civale, R.; Bizzarro, V.; Petrella, A.; Pepe, G.; Campiglia, P.; Russo, P.; Aquino, R.P. In Situ Gelling Alginate-Pectin Blend Particles Loaded with Ac2-26: A New Weapon to Improve Wound Care Armamentarium. Carbohydr. Polym. 2020, 227, 115305. [Google Scholar] [CrossRef] [PubMed]
- Jadach, B.; Świetlik, W.; Froelich, A. Sodium Alginate as a Pharmaceutical Excipient: Novel Applications of a Well-Known Polymer. J. Pharm. Sci. 2022, 111, 1250–1261. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on Modification of Chitosan Biopolymer and Its Potential Applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef] [PubMed]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kou, S.G.; Peters, L.; Mucalo, M. Chitosan: A Review of Molecular Structure, Bioactivities and Interactions with the Human Body and Micro-Organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, S.P.; Ngece, K.; Alven, S.; Aderibigbe, B.A. Gelatin-Based Hybrid Scaffolds: Promising Wound Dressings. Polymers 2021, 13, 2959. [Google Scholar] [CrossRef]
- Yao, C.-H.; Lee, C.-Y.; Huang, C.-H.; Chen, Y.-S.; Chen, K.-Y. Novel Bilayer Wound Dressing Based on Electrospun Gelatin/Keratin Nanofibrous Mats for Skin Wound Repair. Mater. Sci. Eng. C 2017, 79, 533–540. [Google Scholar] [CrossRef]
- Li, T.; Sun, M.; Wu, S. State-of-the-Art Review of Electrospun Gelatin-Based Nanofiber Dressings for Wound Healing Applications. Nanomaterials 2022, 12, 784. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Chitosan, Alginate, Hyaluronic Acid, Gums, and β-Glucan as Potent Adjuvants and Vaccine Delivery Systems for Viral Threats Including SARS-CoV-2: A Review. Int. J. Biol. Macromol. 2021, 182, 1931–1940. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic Acid and Its Biomedical Applications: A Review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Feng, Q.; Wu, D.; Zhao, Y.; Wei, A.; Wei, Q.; Fong, H. Electrospun AOPAN/RC Blend Nanofiber Membrane for Efficient Removal of Heavy Metal Ions from Water. J. Hazard. Mater. 2018, 344, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, M.; Nabavi, S.R.; Emadi, H.; Faraji, M. Development of a Superhydrophilic Nanofiber Membrane for Oil/Water Emulsion Separation via Modification of Polyacrylonitrile/Polyaniline Composite. Polym. Adv. Technol. 2021, 32, 1301–1316. [Google Scholar] [CrossRef]
- Sabantina, L.; Wehlage, D.; Klöcker, M.; Mamun, A.; Grothe, T.; García-Mateos, F.J.; Rodríguez-Mirasol, J.; Cordero, T.; Finsterbusch, K.; Ehrmann, A. Stabilization of Electrospun PAN/Gelatin Nanofiber Mats for Carbonization. J. Nanomater. 2018, 2018, e6131085. [Google Scholar] [CrossRef] [Green Version]
- Jayawickramage, R.A.P.; Balkus, K.J.; Ferraris, J.P. Binder Free Carbon Nanofiber Electrodes Derived from Polyacrylonitrile-Lignin Blends for High Performance Supercapacitors. Nanotechnology 2019, 30, 355402. [Google Scholar] [CrossRef]
- Hou, C.; Yang, H.; Xu, Z.-L.; Wei, Y. Preparation of PAN/PAMAM Blend Nanofiber Mats as Efficient Adsorbent for Dye Removal. Fiber. Polym. 2015, 16, 1917–1924. [Google Scholar] [CrossRef]
- Kim, B.-H.; Yang, K.S.; Woo, H.-G.; Oshida, K. Supercapacitor Performance of Porous Carbon Nanofiber Composites Prepared by Electrospinning Polymethylhydrosiloxane (PMHS)/Polyacrylonitrile (PAN) Blend Solutions. Synth. Met. 2011, 161, 1211–1216. [Google Scholar] [CrossRef]
- Choi, S.Y.; Han, E.M.; Park, K.H. Porosity Control of Electrospun PAN/PMMA Nanofiber Webs. Mol. Cryst. Liq. Cryst. 2019, 688, 68–74. [Google Scholar] [CrossRef]
- Ahmed, R.M. Surface Characterization and Optical Study on Electrospun Nanofibers of PVDF/PAN Blends. Fiber Integr. Opt. 2017, 36, 78–90. [Google Scholar] [CrossRef]
- Shokrollahzadeh, S.; Tajik, S. Fabrication of Thin Film Composite Forward Osmosis Membrane Using Electrospun Polysulfone/Polyacrylonitrile Blend Nanofibers as Porous Substrate. Desalination 2018, 425, 68–76. [Google Scholar] [CrossRef]
- Kim, M.N.; Koh, J.; Lee, Y.; Kim, H. Preparation of PVA/PAN Bicomponent Nanofiber via Electrospinning and Selective Dissolution. J. Appl. Polym. Sci. 2009, 113, 274–282. [Google Scholar] [CrossRef]
- Sun, Z.; Feng, T.; Zhou, Z.; Wu, H. Removal of Methylene Blue in Water by Electrospun PAN/β-CD Nanofibre Membrane. E-Polym. 2021, 21, 398–410. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Qin, D.; Sun, M.; Wang, T.; Chen, X. Research Status of Self-Healing Hydrogel for Wound Management: A Review. Int. J. Biol. Macromol. 2020, 164, 2108–2123. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Song, W.; Xu, L.; Yu, D.-G.; Annie Bligh, S.W. A Review on Electrospun Poly(Amino Acid) Nanofibers and Their Applications of Hemostasis and Wound Healing. Biomolecules 2022, 12, 794. [Google Scholar] [CrossRef]
- Bao, Y.; Tay, Y.S.; Lim, T.-T.; Wang, R.; Webster, R.D.; Hu, X. Polyacrylonitrile (PAN)-Induced Carbon Membrane with in-Situ Encapsulated Cobalt Crystal for Hybrid Peroxymonosulfate Oxidation-Filtration Process: Preparation, Characterization and Performance Evaluation. Chem. Eng. J. 2019, 373, 425–436. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Zhao, J.; Lv, W.; Wu, Q.; Ren, H.; Chen, C.; Sun, D. Liquid-Assisted Electrospinning Three-Dimensional Polyacrylonitrile Nanofiber Crosslinked with Chitosan. J. Nanomater. 2021, 2021, e4639317. [Google Scholar] [CrossRef]
- Yu, D.-G. Preface—Bettering drug delivery knowledge from pharmaceutical techniques and excipients. Curr. Drug Deliv. 2021, 18, 2–3. [Google Scholar] [CrossRef]
- Köse, M.D.; Ungun, N.; Bayraktar, O. Eggshell Membrane Based Turmeric Extract Loaded Orally Disintegrating Films. Curr. Drug Deliv. 2021, 19, 547–559. [Google Scholar] [CrossRef]
- Ortega, C.A.; Favier, L.S.; Cianchino, V.A.; Cifuente, D.A. New Orodispersible Mini Tablets of Enalapril Maleate by Direct Compression for Pediatric Patients. Curr. Drug Deliv. 2020, 17, 505–510. [Google Scholar] [CrossRef]
- Khalid, G.M.; Selmin, F.; Musazzi, U.M.; Gennari, C.G.M.; Minghetti, P.; Cilurzo, F. Trends in the Characterization Methods of Orodispersible Films. Curr. Drug Deliv. 2021, 18, 935–946. [Google Scholar] [CrossRef]
- Akombaetwa, N.; Bwanga, A.; Makoni, P.A.; Witika, B.A. Applications of Electrospun Drug-Eluting Nanofibers in Wound Healing: Current and Future Perspectives. Polymers 2022, 14, 2931. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.N.; Ullah, A.; Haider, M.K.; Hussain, N.; Ullah, S.; Hashmi, M.; Khan, M.Q.; Kim, I.S. Evaluating Antibacterial Efficacy and Biocompatibility of PAN Nanofibers Loaded with Diclofenac Sodium Salt. Polymers 2021, 13, 510. [Google Scholar] [CrossRef] [PubMed]
- Semnani, K.; Shams-Ghahfarokhi, M.; Afrashi, M.; Fakhrali, A.; Semnani, D. Antifungal Activity of Eugenol Loaded Electrospun PAN Nanofiber Mats against Candida Albicans. Curr. Drug Deliv. 2018, 15, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Taymouri, S.; Hashemi, S.; Varshosaz, J.; Minaiyan, M.; Talebi, A. Fabrication and Evaluation of Hesperidin Loaded Polyacrylonitrile/Polyethylene Oxide Nanofibers for Wound Dressing Application. J. Biomater. Sci. Polym. Ed. 2021, 32, 1944–1965. [Google Scholar] [CrossRef]
- Kharaghani, D.; Gitigard, P.; Ohtani, H.; Kim, K.O.; Ullah, S.; Saito, Y.; Khan, M.Q.; Kim, I.S. Design and Characterization of Dual Drug Delivery Based on In-Situ Assembled PVA/PAN Core-Shell Nanofibers for Wound Dressing Application. Sci. Rep. 2019, 9, 12640. [Google Scholar] [CrossRef] [Green Version]
- Fayemi, O.E.; Ekennia, A.C.; Katata-Seru, L.; Ebokaiwe, A.P.; Ijomone, O.M.; Onwudiwe, D.C.; Ebenso, E.E. Antimicrobial and Wound Healing Properties of Polyacrylonitrile-Moringa Extract Nanofibers. ACS Omega 2018, 3, 4791–4797. [Google Scholar] [CrossRef]
- Shen, X.; Yu, D.; Zhang, X.; Branford-White, C.; Zhu, L. Preparation and Characterization of TAM-Loaded HPMC/PAN Composite Fibers for Improving Drug-Release Profiles. J. Biomater. Sci. Polym. Ed. 2011, 22, 2227–2240. [Google Scholar] [CrossRef]
- Wu, H.; Bremner, D.H.; Li, H.; Shi, Q.; Wu, J.; Xiao, R.; Zhu, L. A Novel Multifunctional Biomedical Material Based on Polyacrylonitrile: Preparation and Characterization. Mater. Sci. Eng. C 2016, 62, 702–709. [Google Scholar] [CrossRef] [Green Version]
- Lv, H.; Guo, S.; Zhang, G.; He, W.; Wu, Y.; Yu, D.-G. Electrospun Structural Hybrids of Acyclovir-Polyacrylonitrile at Acyclovir for Modifying Drug Release. Polymers 2021, 13, 4286. [Google Scholar] [CrossRef]
- Yu, D.-G.; Wang, M.; Ge, R. Strategies for Sustained Drug Release from Electrospun Multi-Layer Nanostructures. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1772. [Google Scholar] [CrossRef]
- Lee, H.; Song, C.; Baik, S.; Kim, D.; Hyeon, T.; Kim, D.-H. Device-Assisted Transdermal Drug Delivery. Adv. Drug Deliv. Rev. 2018, 127, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, R.; Raman, S. Recent Trends in Carbon Nanotubes Based Prostate Cancer Therapy: A Biomedical Hybrid for Diagnosis and Treatment. Curr. Drug Deliv. 2021, 19, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Esim, O.; Hascicek, C. Lipid-Coated Nanosized Drug Delivery Systems for an Effective Cancer Therapy. Curr. Drug Deliv. 2021, 18, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Ejeta, F.; Gabriel, T.; Joseph, N.M.; Belete, A. Formulation, Optimization and in Vitro Evaluation of Fast Disintegrating Tablets of Salbutamol Sulphate Using a Combination of Superdisintegrant and Subliming Agent. Curr. Drug Deliv. 2022, 19, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Bigogno, E.R.; Soares, L.; Mews, M.H.R.; Zétola, M.; Bazzo, G.C.; Stulzer, H.K.; Pezzini, B.R. It Is Possible to Achieve Tablets with Good Tabletability from Solid Dispersions—The Case of the High Dose Drug Gemfibrozil. Curr. Drug Deliv. 2021, 18, 460–470. [Google Scholar] [CrossRef]
- Obeidat, W.M.; Al-Natour, M.A. Assessment of Once Daily Controlled-Release Ibuprofen Matrix Tablets Prepared Using Eudragit ®E100/Carbopol® 971p Nf Polymers and Their Salt Combinations. Curr. Drug Deliv. 2022, 19, 74–85. [Google Scholar] [CrossRef]
- Sultana, M.; Sultana, S.; Hussain, K.; Saeed, T.; Butt, M.A.; Raza, S.A.; Mahmood, R.; Hassan, S.; Anwer, U.U.; Bukhari, N.I. Enhanced Mefenamic Acid Release from Poloxamer-Silicon Dioxide Gel Filled in Hard Gelatin Capsules—An Application of Liquid Semisolid Matrix Technology for Insoluble Drug. Curr. Drug Deliv. 2021, 19, 801–811. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, H.; Liu, Y.; Gao, Y.; Kim, H.Y.; Ouyang, Y.; Yu, D.-G. Progresses on Electrospun Metal–Organic Frameworks Nanofibers and Their Wastewater Treatment Applications. Mater. Today Chem. 2022, 25, 100974. [Google Scholar] [CrossRef]
- Ringaci, A.; Yaremenko, A.V.; Shevchenko, K.G.; Zvereva, S.D.; Nikitin, M.P. Metal-Organic Frameworks for Simultaneous Gene and Small Molecule Delivery in Vitro and in Vivo. Chem. Eng. J. 2021, 418, 129386. [Google Scholar] [CrossRef]
- Yang, J.; Liu, C.-L.; Ding, Y.-N.; Sun, T.-C.; Bai, X.-H.; Cao, Z.-K.; Ramakrishna, S.; Zhang, J.; Long, Y.-Z. Synergistic Antibacterial Polyacrylonitrile/Gelatin Nanofibers Coated with Metal-Organic Frameworks for Accelerating Wound Repair. Int. J. Biol. Macromol. 2021, 189, 698–704. [Google Scholar] [CrossRef]
- Khodayari, P.; Jalilian, N.; Ebrahimzadeh, H.; Amini, S. Electrospun Cellulose Acetate/Polyacrylonitrile/Thymol/Mg-Metal Organic Framework Nanofibers as Efficient Sorbent for Pipette-Tip Micro-Solid Phase Extraction of Anti-Cancer Drugs. React. Funct. Polym. 2022, 173, 105217. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, T.; Qiu, Q.; Qin, X. Quaternary Ammonium Salt–Modified Polyacrylonitrile/Polycaprolactone Electrospun Nanofibers with Enhanced Antibacterial Properties. Text. Res. J. 2021, 91, 2194–2203. [Google Scholar] [CrossRef]
- Qiu, Q.; Wu, J.; Quan, Z.; Zhang, H.; Qin, X.; Wang, R.; Yu, J. Electrospun Nanofibers of Polyelectrolyte–Surfactant Complexes for Antibacterial Wound Dressing Application. Soft Matter 2019, 15, 10020–10028. [Google Scholar] [CrossRef]
- Garcia Cervantes, M.Y.; Han, L.; Kim, J.; Chitara, B.; Wymer, N.; Yan, F. N-Halamine-Decorated Electrospun Polyacrylonitrile Nanofibrous Membranes: Characterization and Antimicrobial Properties. React. Funct. Polym. 2021, 168, 105058. [Google Scholar] [CrossRef]
- Ullah, S.; Hashmi, M.; Kharaghani, D.; Khan, M.Q.; Saito, Y.; Yamamoto, T.; Lee, J.; Kim, I.S. Antibacterial Properties of in Situ and Surface Functionalized Impregnation of Silver Sulfadiazine in Polyacrylonitrile Nanofiber Mats. Int. J. Nanomed. 2019, 14, 2693–2703. [Google Scholar] [CrossRef] [Green Version]
- Bowler, P.G. Antibiotic Resistance and Biofilm Tolerance: A Combined Threat in the Treatment of Chronic Infections. J. Wound Care 2018, 27, 273–277. [Google Scholar] [CrossRef]
- Negut, I.; Bita, B.; Groza, A. Polymeric Coatings and Antimicrobial Peptides as Efficient Systems for Treating Implantable Medical Devices Associated-Infections. Polymers 2022, 14, 1611. [Google Scholar] [CrossRef]
- Ardita, N.F.; Mithasari, L.; Untoro, D.; Salasia, S.I.O. Potential Antimicrobial Properties of the Ulva Lactuca Extract against Methicillin-Resistant Staphylococcus Aureus-Infected Wounds: A Review. Vet. World 2021, 14, 1116–1123. [Google Scholar] [CrossRef]
- Fontana, K.; Ventimiglia, L.; Mutus, B. Nitric Oxide Generating Copper–Chitosan Particles for Wound Healing Applications. J. Chem. Technol. Biotechnol. 2018, 93, 2093–2101. [Google Scholar] [CrossRef]
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric Oxide Therapy for Diabetic Wound Healing. Adv. Healthc. Mater. 2019, 8, 1801210. [Google Scholar] [CrossRef]
- Chavhan, M.M.; Gadhave, R.V.; Ozarde, Y.S.; Choudhari, G.B. Therapeutic Role of Nitric Oxide in Diabetic Wound Healing: A Systematic Review. J. Pharm. Res. Int. 2021, 33, 68–80. [Google Scholar] [CrossRef]
- Workman, C.D.; Hopkins, S.; Pant, J.; Goudie, M.; Handa, H. Covalently Bound S-Nitroso-N-Acetylpenicillamine to Electrospun Polyacrylonitrile Nanofibers for Multifunctional Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2021, 7, 5279–5287. [Google Scholar] [CrossRef] [PubMed]
- Ning, T.; Zhou, Y.; Xu, H.; Guo, S.; Wang, K.; Yu, D.-G. Orodispersible Membranes from a Modified Coaxial Electrospinning for Fast Dissolution of Diclofenac Sodium. Membranes 2021, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Mohamady Hussein, M.A.; Guler, E.; Rayaman, E.; Cam, M.E.; Sahin, A.; Grinholc, M.; Sezgin Mansuroglu, D.; Sahin, Y.M.; Gunduz, O.; Muhammed, M. Dual-Drug Delivery of Ag-Chitosan Nanoparticles and Phenytoin via Core-Shell PVA/PCL Electrospun Nanofibers. Carbohydr. Polym. 2021, 270, 118373. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, G.; Zhao, Y.; Zhou, M.; Zhong, A.; Sun, J. Promotion of Skin Regeneration through Coaxial Electrospun Fibers Loaded with Basic Fibroblast Growth Factor. Adv. Compos. Hybrid Mater. 2022, 5, 1111–1125. [Google Scholar] [CrossRef]
- Ghazalian, M.; Afshar, S.; Rostami, A.; Rashedi, S.; Bahrami, S.H. Fabrication and Characterization of Chitosan-Polycaprolactone Core-Shell Nanofibers Containing Tetracycline Hydrochloride. Colloids Surf. A 2022, 636, 128163. [Google Scholar] [CrossRef]
- El-Newehy, M.H.; El-Naggar, M.E.; Alotaiby, S.; El-Hamshary, H.; Moydeen, M.; Al-Deyab, S. Preparation of Biocompatible System Based on Electrospun CMC/PVA Nanofibers as Controlled Release Carrier of Diclofenac Sodium. J. Macromol. Sci. A 2016, 53, 566–573. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Yu, D.-G.; Liu, H.; Liu, Y.; Liu, P. Electrospun PVP-Core/PHBV-Shell Fibers to Eliminate Tailing off for an Improved Sustained Release of Curcumin. Mol. Pharm. 2021, 18, 4170–4178. [Google Scholar] [CrossRef]
- Han, L.; Ma, Y.; Dou, H.; Fan, W. Dual-Functional SFP/PAN Based Nano Drug Release System for Treatment and Nutrients. Text. Res. J. 2021, 91, 1742–1751. [Google Scholar] [CrossRef]
- Li, R.; Cheng, Z.; Yu, X.; Wang, S.; Han, Z.; Kang, L. Preparation of Antibacterial PCL/PVP-AgNP Janus Nanofibers by Uniaxial Electrospinning. Mater. Lett. 2019, 254, 206–209. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, R.; Chen, L.; Sun, R.; Zhang, Y.; Sheng, R.; Du, T.; Li, Y.; Qi, Y. A Multifunctional Janus Electrospun Nanofiber Dressing with Biofluid Draining, Monitoring, and Antibacterial Properties for Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 12984–13000. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Long, Y.Z.; Zhang, H.D.; Li, M.M.; Duvail, J.L.; Jiang, X.Y.; Yin, H.L. Advances in Three-Dimensional Nanofibrous Macrostructures via Electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Uttayarat, P.; Perets, A.; Li, M.; Pimton, P.; Stachelek, S.J.; Alferiev, I.; Composto, R.J.; Levy, R.J.; Lelkes, P.I. Micropatterning of Three-Dimensional Electrospun Polyurethane Vascular Grafts. Acta Biomater. 2010, 6, 4229–4237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chang, J. Electrospinning of Three-Dimensional Nanofibrous Tubes with Controllable Architectures. Nano Lett. 2008, 8, 3283–3287. [Google Scholar] [CrossRef] [PubMed]
- Hani, N.M.; Torkamani, A.E.; Azarian, M.H.; Mahmood, K.W.; Ngalim, S.H. Characterisation of Electrospun Gelatine Nanofibres Encapsulated with Moringa Oleifera Bioactive Extract. J. Sci. Food Agric. 2017, 97, 3348–3358. [Google Scholar] [CrossRef]
- Jaja-Chimedza, A.; Zhang, L.; Wolff, K.; Graf, B.L.; Kuhn, P.; Moskal, K.; Carmouche, R.; Newman, S.; Salbaum, J.M.; Raskin, I. A Dietary Isothiocyanate-Enriched Moringa (Moringa Oleifera) Seed Extract Improves Glucose Tolerance in a High-Fat-Diet Mouse Model and Modulates the Gut Microbiome. J. Funct. Foods 2018, 47, 376–385. [Google Scholar] [CrossRef]
- Shirazi, M.M.A.; Bazgir, S.; Meshkani, F. A Dual-Layer, Nanofibrous Styrene-Acrylonitrile Membrane with Hydrophobic/Hydrophilic Composite Structure for Treating the Hot Dyeing Effluent by Direct Contact Membrane Distillation. Chem. Eng. Res. Des. 2020, 164, 125–146. [Google Scholar] [CrossRef]
- Afghah, F.; Dikyol, C.; Altunbek, M.; Koc, B. Biomimicry in Bio-Manufacturing: Developments in Melt Electrospinning Writing Technology towards Hybrid Biomanufacturing. Appl. Sci. 2019, 9, 3540. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Curtis, C.K.; Thoppey, N.M.; Bochinski, J.R.; Gorga, R.E.; Clarke, L.I. Unconfined, Melt Edge Electrospinning from Multiple, Spontaneous, Self-Organized Polymer Jets. Mater. Res. Express 2014, 1, 045304. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, F.; Wang, M.; Lv, H.; Yu, D.-G.; Liu, X.; Shen, H. Electrospun Hierarchical Structural Films For Effective Wound Healing. Biomater. Adv. 2022, 136, 212795. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Yang, Z.; Chen, X.; Yu, D.-G.; Shao, J. Hybrid Films Prepared from a Combination of Electrospinning and Casting for Offering a Dual-phase Drug Release. Polymers 2022, 14, 2132. [Google Scholar] [CrossRef]
- Ghosal, K.; Augustine, R.; Zaszczynska, A.; Barman, M.; Jain, A.; Hasan, A.; Kalarikkal, N.; Sajkiewicz, P.; Thomas, S. Novel Drug Delivery Systems Based on Triaxial Electrospinning Based Nanofibers. React. Funct. Polym. 2021, 163, 104895. [Google Scholar] [CrossRef]
- Tang, Y.; Varyambath, A.; Ding, Y.; Chen, B.; Huang, X.; Zhang, Y.; Yu, D.G.; Kim, I.; Song, W. Porous organic polymers for drug delivery: Hierarchical pore structures, variable morphologies, and biological properties. Biomater. Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.; Gao, Y.; Liu, Y.; Yu, D.; Liu, P. Electrospun Core—Sheath Nanofibers with Variable Shell Thickness for Modifying Curcumin Release to Achieve a Better Antibacterial Performance. Biomolecules 2022, 12, 1057. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Che, S.; Feng, Y.; Guan, L.; Yang, X.; Zhao, Y.; Wang, J.; Zvyagin, A.V.; Yang, B. A Smart Hydrogel Patch with High Transparency, Adhesiveness and Hemostasis for All-Round Treatment and Glucose Monitoring of Diabetic Foot Ulcers. J. Mater. Chem. B 2022, 10, 5804–5817. [Google Scholar] [CrossRef]
- Krysiak, Z.J.; Stachewicz, U. Electrospun fibers as carriers for topical drug delivery and release in skin bandages and patches for atopic dermatitis treatment. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1829. [Google Scholar] [CrossRef] [PubMed]












| Polymers Blended with PAN | Solvent | Mean Fiber Diameter (nm) | Ref. |
|---|---|---|---|
| Cellulose acetate | DMF | 200–500 | [172] |
| Polyaniline | DMF | 87–190 | [173] |
| Cellulose acetate butyrate | DMF/acetone | 883 | [150] |
| GEL | DMSO | / | [174] |
| Lignin | DMF | 70.77–333.75 | [175] |
| Polyamidoamine | DMF | 200–500 | [176] |
| Polymethylhydrosiloxane | DMF | / | [177] |
| Poly (methyl methacrylate) | DMF | / | [178] |
| Poly(vinylidene fluoride) | DMF | 745–825 | [179] |
| Polysulfone | 1-methyl-2-pyrrolidone/DMF | 250–500 | [180] |
| Poly(vinylalcohol) | DMSO | / | [181] |
| β-cyclodextrin | DMF | 280–680 | [182] |
| Drug | Solvent | Advantages | Ref. |
|---|---|---|---|
| Diclofenac sodium | DMF | At 6% density, the Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) inhibition zones were shown to be 16 ± 0.46 mm and 15.5 ± 0.28 mm, respectively. DLF-loaded nanofibers showed better cell viability and the dressing still had good biocompatibility. | [192] |
| Eugenol | / | In vitro antibacterial activity against C. albicans. With the effect of analgesic | [193] |
| Hesperidin | DMF | With the effects of antibacterial, anti-inflammatory, antioxidant, and angiogenic. It has shown in vitro that when a one-centimeter-diameter wound on the back of a rat was treated with Hesperidin-loaded nanofibers, Hesperidin-free nanofibers, and normal saline, the former wound closure was significantly faster than the latter two. | [194] |
| Gentamine sulfate | DMF | An antibiotic drug. Reduces inflammation and promotes wound regeneration. | [195] |
| Moringa extract | Ethanol/DMF | It has an effective inhibitory effect on multidrug-resistant methicillin-resistant Staphylococcus aureus. In 16 wt% PAN nanofibers, the higher the concentration of Moringa oleifera extract, the better the antibacterial activity against Escherichia coli and Staphylococcus aureus. | [196] |
| Tamoxifen | DMF | In clinical medicine, it is used for the prevention and treatment of advanced breast cancer. | [197] |
| Curcumin | DMF | The hybrid spun fibers containing Cur have enhanced mechanical properties and biocompatibility, and the Cur is distributed within the fibers in an amorphous state. | [198] |
| Vitamin E acetate | DMF | Fat-soluble antioxidants. Hybrid spun fibers with vitamin E acetate have enhanced mechanical properties and biocompatibility. | [198] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Xu, X.; Fu, J.; Yu, D.-G.; Liu, Y. Recent Progress in Electrospun Polyacrylonitrile Nanofiber-Based Wound Dressing. Polymers 2022, 14, 3266. https://doi.org/10.3390/polym14163266
Huang C, Xu X, Fu J, Yu D-G, Liu Y. Recent Progress in Electrospun Polyacrylonitrile Nanofiber-Based Wound Dressing. Polymers. 2022; 14(16):3266. https://doi.org/10.3390/polym14163266
Chicago/Turabian StyleHuang, Chang, Xizi Xu, Junhao Fu, Deng-Guang Yu, and Yanbo Liu. 2022. "Recent Progress in Electrospun Polyacrylonitrile Nanofiber-Based Wound Dressing" Polymers 14, no. 16: 3266. https://doi.org/10.3390/polym14163266