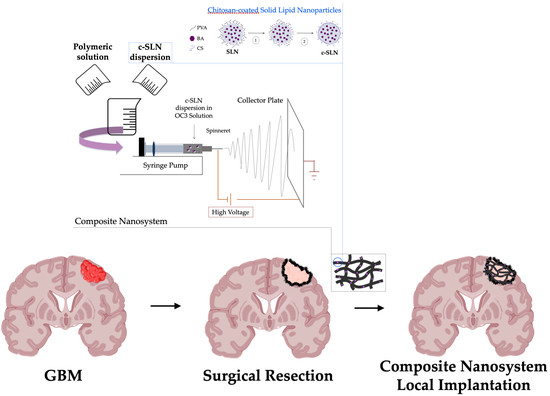
A Composite Nanosystem as a Potential Tool for the Local Treatment of Glioblastoma: Chitosan-Coated Solid Lipid Nanoparticles Embedded in Electrospun Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Solid Lipid Nanoparticles
2.3. Preparation of Chitosan-Coated SLN (c-SLN)
2.4. Characterization of SLN and c-SLN
2.4.1. Particle Size, Size Distribution, and Zeta Potential
2.4.2. Storage Stability Studies
2.4.3. Morphological Analysis
2.4.4. Production Yield
2.4.5. Encapsulation Efficiency
2.4.6. Cytotoxicity Test
2.4.7. Cell Uptake
2.5. Preparation of Electrospun Nanofibers (NFs)
2.5.1. Preparation of Polymeric Solutions for Electrospinning
2.5.2. Electrospinning and Nanofiber Characterization
2.6. Preparation and Characterization of Composite Nanosystem (CN)
2.7. Statystical Analysis
3. Results and Discussion
3.1. Chitosan-Coated Solid Lipid Nanoparticles (c-SLN)
3.2. Electrsospun Nanofibers (NFs)
3.3. Composite Nanosystem (NC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BA | behenic acid |
| BA-SLN | behenic acid-based solid lipid nanoparticles |
| c-BA-SLN | chitosan-coated behenic acid-based solid lipid nanoparticles |
| CN | composite nanosystem |
| CS | chitosan |
| c-SLN | chitosan-coated solid lipid nanoparticles |
| DLS | dynamic light scattering |
| EE% | encapsulation efficiency |
| ELS | electrophoretic light scattering |
| GBM | glioblastoma multiforme |
| HCS | high molecular weight chitosan |
| HD | hydrolysis degree |
| h-PEO | high molecular weight poly(ethylene oxide) |
| LCS | low molecular weight chitosan |
| MCS | medium molecular weight chitosan |
| MW | molecular weight |
| Na-BA | sodium-behenate (or behenic acid sodium salt) |
| Na-SA | sodium-stearate (or stearic acid sodium salt) |
| NFs | nanofibers |
| NR | Nile Red |
| NR/BA-SLN | Nile Red-loaded behenic acid-based solid lipid nanoparticles |
| NR/c-BA-SLN | Nile Red-loaded chitosan-coated behenic acid-based solid lipid nanoparticles |
| O-CMCS | O-carboxymethyl chitosan |
| P407 | Poloxamer P407 |
| PDI | polydispersity index |
| PVA | poly(vinyl alcohol) |
| SA | stearic acid |
| SA-SLN | stearic acid-based solid lipid nanoparticles |
| SLN | solid lipid nanoparticles |
| TEM | transmission electron microscope |
| WHO | World Health Organization |
| Y% | production yield |
References
- Adamson, C.; Kanu, O.O.; Mehta, A.I.; Di, C.; Lin, N.; Mattox, A.K.; Bigner, D.D. Glioblastoma multiforme: A review of where we have been and where we are going. Expert Opin. Investig. Drugs 2009, 18, 1061–1083. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osuka, S.; Van Meir, E.G. Overcoming therapeutic resistance in glioblastoma: The way forward. J. Clin. Investig. 2017, 127, 415–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, O.G.; Brzozowski, J.S.; Skelding, K.A. Glioblastoma Multiforme: An Overview of Emerging Therapeutic Targets. Front. Oncol. 2019, 9, 963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; van Straten, D.; Broekman, M.D.L.; Préat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355–1372. [Google Scholar] [CrossRef]
- Norouzi, M. Recent advances in brain tumor therapy: Application of electrospun nanofibers. Drug Discov. Today 2018, 23, 912–919. [Google Scholar] [CrossRef]
- Ranganath, S.H.; Wang, C. Biodegradable microfiber implants delivering paclitaxel for post-surgical chemotherapy against malignant glioma. Biomaterials 2008, 29, 2996–3003. [Google Scholar] [CrossRef]
- Ranganath, S.H.; Fu, Y.; Arifin, D.Y.; Kee, I.; Zheng, L.; Lee, H.; Chow, P.K.; Wang, C. The use of submicron/nanoscale PLGA implants to deliver paclitaxel with enhanced pharmacokinetics and therapeutic efficacy in intracranial glioblastoma in mice. Biomaterials 2010, 31, 5199–5207. [Google Scholar] [CrossRef] [PubMed]
- Irani, M.; Sadeghi, G.M.M.; Haririan, I. Electrospun biocompatible poly (ε-caprolactonediol)-based polyurethane core/shell nanofibrous scaffold for controlled release of temozolomide. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 361–366. [Google Scholar] [CrossRef]
- Tavakoli, R.; Vakilian, S.; Jamshidi-Adegani, F.; Sharif, S.; Ardeshirylajimi, A.; Soleimani, M. Prolonged drug release using PCL–TMZ nanofibers induce the apoptotic behavior of U87 glioma cells. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 873–878. [Google Scholar] [CrossRef]
- Musiał-Kulik, M.; Włodarczyk, J.; Stojko, M.; Karpeta-Jarząbek, P.; Pastusiak, M.; Kasperczyk, J. Bioresorbable, electrospun nonwoven for delayed and prolonged release of temozolomide and nimorazole. Eur. J. Pharm. Biopharm. 2021, 161, 29–36. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Xu, X.; Lu, T.; Wang, X.; Yang, L.; Jing, X. BCNU-loaded PEG-PLLA ultrafine fibers and their in vitro antitumor activity against Glioma C6 cells. J. Control. Release 2006, 114, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.; Liao, J.; Chen, W.; Kao, Y.; Liu, S. Sustainable release of carmustine from biodegradable poly [(d,L)-lactide-co-glycolide] nanofibrous membranes in the cerebral cavity: In vitro and in vivo studies. Expert Opin. Drug Deliv. 2013, 10, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Irani, M.; Sadeghi, G.M.M.; Haririan, I. A novel biocompatible drug delivery system of chitosan/temozolomide nanoparticles loaded PCL-PU nanofibers for sustained delivery of temozolomide. Int. J. Biol. Macromol. 2017, 97, 744–751. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Hyaluronic acid and chitosan-based nanosystems: A new dressing generation for wound care. Expert Opin. Drug Deliv. 2019, 16, 715–740. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.X.; Chen, L.; Wang, W.; Jia, Y.; Chang, A.; Mo, X.; Wang, H.; He, C. Electrospun nanofibers incorporating self-decomposable silica nanoparticles as carriers for controlled delivery of anticancer drug. RSC Adv. 2015, 5, 65897–65904. [Google Scholar] [CrossRef]
- Chen, J.M.; Gao, S.; Dong, M.; Song, J.; Yang, C.; Howard, K.A.; Kjems, J.; Besenbacher, F. Chitosan/siRNA nanoparticles encapsulated in PLGA nanofibers for siRNA delivery. ACS Nano 2012, 6, 4835–4844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Xing, R.; Liu, S.; Li, K.; Qin, Y.; Yu, H.; Li, P. Comparison in docetaxel-loaded nanoparticles based on three different carboxymethyl chitosans. Int. J. Biol. Macromol. 2017, 101, 1012–1018. [Google Scholar] [CrossRef]
- Key, J.; Park, K. Multicomponent, Tumor-Homing Chitosan Nanoparticles for Cancer Imaging and Therapy. Int. J. Mol. Sci. 2017, 18, 594. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R.V. Chitosan as a coating material for nanoparticles intended for biomedical applications. React. Funct. Polym. 2020, 147, 104459. [Google Scholar] [CrossRef]
- Aldea, M.; Potara, M.; Soritau, O.; Florian, I.S.; Florea, A.; Nagy-Simon, T.; Pileczki, V.; Brie, V.; Maniu, D.; Kacso, G. Chitosan-capped gold nanoparticles impair radioresistant glioblastoma stem-like cells. J. BUON 2018, 23, 800–813. [Google Scholar] [CrossRef] [PubMed]
- Eslahi, M.; Dana, P.M.; Asemi, Z.; Hallajzadeh, J.; Mansournia, M.A.; Yousefi, B. The effects of chitosan-based materials on glioma: Recent advances in its applications for diagnosis and treatment. Int. J. Biol. Macromol. 2021, 168, 124–129. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Chorilli, M. An overview of carboxymethyl derivatives of chitosan: Their use as biomaterials and drug delivery systems. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1349–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battaglia, L.; Gallarate, M.; Peira, E.; Chirio, D.; Muntoni, E.; Biasibetti, E.; Capucchio, M.T.; Valazza, A.; Panciani, P.P.; Lanotte, M.; et al. Solid lipid nanoparticles for potential doxorubicin delivery in glioblastoma treatment: Preliminary in vitro studies. J. Pharm. Sci. 2014, 103, 2157–2165. [Google Scholar] [CrossRef]
- Ramalingam, P.; Yoo, S.W.; Ko, Y.T. Nanodelivery systems based on mucoadhesive polymer coated solid lipid nanoparticles to improve the oral intake of food curcumin. Food Res. Int. 2016, 84, 113–119. [Google Scholar] [CrossRef]
- Rossi, S.; Vigani, B.; Puccio, A.; Bonferoni, M.C.; Sandri, G.; Ferrari, F. Chitosan Ascorbate Nanoparticles for the Vaginal Delivery of Antibiotic Drugs in Atrophic Vaginitis. Mar. Drugs 2017, 15, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battaglia, L.; Gallarate, M.; Cavalli, R.; Trotta, M. Solid lipid nanoparticles produced through a coacervation method. J. Microencapsul. 2010, 27, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 6. [Google Scholar] [CrossRef]
- Farag, R.K.; Mohamed, R.R. Synthesis and characterization of carboxymethyl chitosan nanogels for swelling studies and antimicrobial activity. Molecules 2012, 18, 190–203. [Google Scholar] [CrossRef]
- Hao, J.; Wang, F.; Wang, X.; Zhang, D.; Bi, Y.; Gao, Y.; Zhao, X.; Zhang, Q. Development and optimization of baicalin-loaded solid lipid nanoparticles prepared by coacervation method using central composite design. Eur. J. Pharm. Sci. 2012, 47, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Chirio, D.; Gallarate, M.; Peira, E.; Battaglia, L.; Muntoni, E.; Riganti, C.; Biasibetti, E.; Capucchio, M.T.; Valazza, A.; Panciani, P.; et al. Positive-charged solid lipid nanoparticles as paclitaxel drug delivery system in glioblastoma treatment. Eur. J. Pharm. Biopharm. 2014, 88, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Xu, X.; Feng, J.; Liu, M.; Hu, K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int. J. Pharm. 2019, 560, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Wang, H.; Han, J.; Zhao, S.; Dumbleton, J.; Agarwal, P.; Zhang, W.; Zhao, G.; Yu, J.; Zynger, D.L.; et al. Chitosan-Decorated Doxorubicin-Encapsulated Nanoparticle Targets and Eliminates Tumor Reinitiating Cancer Stem-like Cells. ACS Nano 2015, 9, 5725–5740. [Google Scholar] [CrossRef]
- Martins, S.; Costa-Lima, S.; Carneiro, T.; Cordeiro-da-Silva, A.; Souto, E.B.; Ferreira, D.C. Solid lipid nanoparticles as intracellular drug transporters: An investigation of the uptake mechanism and pathway. Int. J. Pharm. 2012, 430, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [Green Version]
- Rogina, A. Electrospinning process: Versatile preparation method for biodegradable and natural polymers and biocomposite systems applied in tissue engineering and drug delivery. Appl. Surf. Sci. 2014, 296, 221–230. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Milanesi, G.; Bruni, G.; Ferrari, F. Coated electrospun alginate-containing fibers as novel delivery systems for regenerative purposes. Int. J. Nanomed. 2018, 13, 6531–6550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigani, B.; Rossi, S.; Milanesi, G.; Bonferoni, M.C.; Sandri, G.; Bruni, G.; Ferrari, F. Electrospun Alginate Fibers: Mixing of Two Different Poly(ethylene oxide) Grades to Improve Fiber Functional Properties. Nanomaterials 2018, 8, 971. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Jeong, L.; Kang, Y.O.; Lee, S.J.; Park, W.H. Electrospinning of polysaccharides for regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Fouda, M.M.G.; El-Aassar, M.R.; Al-Deyab, S.S. Antimicrobial activity of carboxymethyl chitosan/polyethylene oxide nanofibers embedded silver nanoparticles. Carbohydr. Polym. 2013, 92, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Li, X.; Wang, X.; Yan, X.; Yu, M.; Ma, J.; Zhou, Y.; Ramakrishna, S.; Long, Y. Electrospinning of Carboxymethyl Chitosan/Polyoxyethylene Oxide Nanofibers for Fruit Fresh-Keeping. Nanoscale Res. Lett. 2018, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- Farboudi, A.; Mahboobnia, K.; Chogan, F.; Karimi, M.; Askari, A.; Banihashem, S.; Davaran, S.; Irani, M. UiO-66 metal organic framework nanoparticles loaded carboxymethyl chitosan/poly ethylene oxide/polyurethane core-shell nanofibers for controlled release of doxorubicin and folic acid. Int. J. Biol. Macromol. 2020, 150, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Valentino, C.; Cavalloro, V.; Catenacci, L.; Sorrenti, M.; Sandri, G.; Bonferoni, M.C.; Bozzi, C.; Collina, S.; Rossi, S.; et al. Gellan-Based Composite System as a Potential Tool for the Treatment of Nervous Tissue Injuries: Cross-Linked Electrospun Nanofibers Embedded in a RC-33-Loaded Freeze-Dried Matrix. Pharmaceutics 2021, 13, 164. [Google Scholar] [CrossRef] [PubMed]








| Formulation | Na-SA | PVA |
|---|---|---|
| S2-1 | 1 | 2 |
| S3-1 | 1 | 3 |
| Formulation | Na-BA | PVA |
|---|---|---|
| B1-075 | 0.75 | 1 |
| B2-075 | 0.75 | 2 |
| B3-075 | 0.75 | 3 |
| Solution | O-CMCS | h-PEO | P407 |
|---|---|---|---|
| OC1 | 0.25 | 1.5 | 2 |
| OC2 | 0.50 | 1.5 | 2 |
| OC3 | 0.75 | 1.5 | 2 |
| OC4 | 1.0 | 1.5 | 2 |
| OC5 | 1.5 | 1.5 | 2 |
| Formulation | Time 0 | 7 Days after Preparation | ||||
|---|---|---|---|---|---|---|
| Particle Size (nm) | PDI | Zeta Potential (mV) | Particle Size (nm) | PDI | Zeta Potential (mV) | |
| S2-1 | 309.03 ± 13.85 a | 0.039 ± 0.009 f | −2.17 ± 0.21 k | 326.55 ± 17.16 a’ | 0.100 ± 0.029 f’ | −5.68 ± 0.25 k’ |
| S3-1 | 363.78 ± 31.73 b | 0.109 ± 0.025 g | −4.44 ± 0.27 l | 338.25 ± 19.18 b’ | 0.079 ± 0.029 g’ | −8.09 ± 0.27 l’ |
| B1-075 | 336.58 ± 5.69 c | 0.149 ± 0.022 h | −6.05 ± 0.34 m | 323.62 ± 5.85 c’ | 0.117 ± 0.027 h’ | −6.79 ± 0.38 m’ |
| B2-075 | 272.20 ± 10.44 d | 0.197 ± 0.044 i | −4.00 ± 0.59 n | 271.87 ± 2.09 d’ | 0.029 ± 0.019 i’ | −6.61 ± 0.61 n’ |
| B3-075 | 241.82 ± 5.94 e | 0.208 ± 0.055 j | −6.15 ± 0.56 o | 268.20 ± 5.79 e’ | 0.065 ± 0.028 j’ | −2.85 ± 0.22 o’ |
| Coating Method | Formulation | CS MW | Particle Size [nm] | PDI | Zeta Potential [mV] |
|---|---|---|---|---|---|
| BA-SLN | 241.82 ± 5.94 a | 0.208 ± 0.055 | −6.15 ± 0.56 a’’ | ||
| One-step | c-BA-SLN | low | 643.63 ± 134.25 b | 0.297 ± 0.116 | 31.79 ± 2.12 b’’ |
| Two-step | c-BA-SLN | low | 330.40 ± 2.59 c | 0.144 ± 0.026 | 3.11 ± 0.31 c’’ |
| Two-step | c-BA-SLN | medium | 457.00 ± 6.40 d | 0.277 ± 0.191 | 36.97 ± 3.19 d’’ |
| Two-step | c-BA-SLN | high | 460.60 ± 5.39 e | 0.114 ± 0.048 | 45.64 ± 7.38 e’’ |
| Fibers | Diameter (μm) |
|---|---|
| OC1 | 0.57 ± 0.04 a |
| OC2 | 0.70 ± 0.03 b |
| OC3 | 0.85 ± 0.03 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigani, B.; Valentino, C.; Sandri, G.; Listro, R.; Fagiani, F.; Collina, S.; Lanni, C.; Bonferoni, M.C.; Caramella, C.M.; Rossi, S.; et al. A Composite Nanosystem as a Potential Tool for the Local Treatment of Glioblastoma: Chitosan-Coated Solid Lipid Nanoparticles Embedded in Electrospun Nanofibers. Polymers 2021, 13, 1371. https://doi.org/10.3390/polym13091371
Vigani B, Valentino C, Sandri G, Listro R, Fagiani F, Collina S, Lanni C, Bonferoni MC, Caramella CM, Rossi S, et al. A Composite Nanosystem as a Potential Tool for the Local Treatment of Glioblastoma: Chitosan-Coated Solid Lipid Nanoparticles Embedded in Electrospun Nanofibers. Polymers. 2021; 13(9):1371. https://doi.org/10.3390/polym13091371
Chicago/Turabian StyleVigani, Barbara, Caterina Valentino, Giuseppina Sandri, Roberta Listro, Francesca Fagiani, Simona Collina, Cristina Lanni, Maria Cristina Bonferoni, Carla M. Caramella, Silvia Rossi, and et al. 2021. "A Composite Nanosystem as a Potential Tool for the Local Treatment of Glioblastoma: Chitosan-Coated Solid Lipid Nanoparticles Embedded in Electrospun Nanofibers" Polymers 13, no. 9: 1371. https://doi.org/10.3390/polym13091371