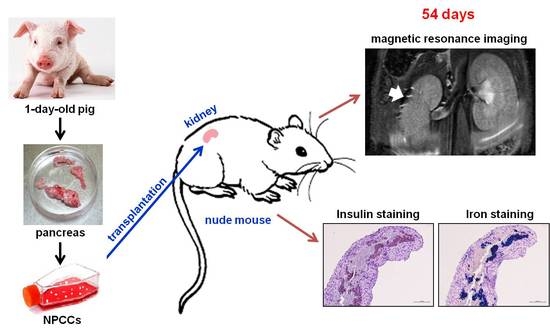
Magnetic Resonance Imaging of Transplanted Porcine Neonatal Pancreatic Cell Clusters Labeled with Chitosan-Coated Superparamagnetic Iron Oxide Nanoparticles in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CSPIO
2.3. Animals
2.4. Preparation and Culture of NPCCs
2.5. Uptake of CSPIO Nanoparticles by NPCCs
2.6. In Vitro MR Scanning
2.7. Transplantation of NPCCs
2.8. In Vivo MR Scanning
2.9. Histological Study of Grafts
2.10. Statistical Analysis
3. Results and Discussion
3.1. Uptake of CSPIO Nanoparticles by NPCCs
3.2. In Vitro MR Image of NPCCs
3.3. In Vivo MR Images of NPCCs after Transplantation
3.4. Histological Studies of NPCC Grafts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.A.; Paty, B.W.; Senior, P.A.; Bigam, D.; Alfadhli, E.; Kneteman, N.M.; Lakey, J.R.; Shapiro, A.M. Five-year follow-up after clinical islet transplantation. Diabetes 2005, 54, 2060–2069. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, A.J.; Ricordi, C.; Hering, B.J.; Auchincloss, H.; Lindblad, R.; Robertson, R.P.; Secchi, A.; Brendel, M.D.; Berney, T.; Brennan, D.C.; et al. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 2006, 355, 1318–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vantyghem, M.C.; de Koning, E.J.P.; Pattou, F.; Rickels, M.R. Advances in β-cell replacement therapy for the treatment of type 1 diabetes. Lancet 2019, 394, 1274–1285. [Google Scholar] [CrossRef]
- Bellin, M.D.; Dunn, T.B. Transplant strategies for type 1 diabetes: Whole pancreas, islet and porcine beta cell therapies. Diabetologia 2020, 63, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Weir, G.C.; Quickel, R.R.; Yoon, K.H.; Tatarkiewicz, K.; Ulrich, T.R.; Hollister-Lock, J.; Bonner-Weir, S. Porcine neonatal pancreatic cell clusters (NPCCs): A potential source of tissue for islet transplantat. Ann. Transplant. 1997, 2, 63–68. [Google Scholar]
- Milner, R.D.; Ashworth, M.A.; Barson, A.J. Insulin release from human foetal pancreas in response to glucose, leucine and arginine. J. Endocrinol. 1972, 52, 497–505. [Google Scholar] [CrossRef]
- Korbutt, G.S.; Elliott, J.F.; Ao, Z.; Smith, D.K.; Warnock, G.L.; Rajotte, R.V. Large scale isolation, growth, and function of porcine neonatal islet cells. J. Clin. Investig. 1996, 97, 2119–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, K.H.; Quickel, R.R.; Tatarkiewicz, K.; Ulrich, T.R.; Hollister-Lock, J.; Trivedi, N.; Bonner-Weir, S.; Weir, G.C. Differentiation and expansion of beta cell mass in porcine neonatal pancreatic cell clusters transplanted into nude mice. Cell Transplant. 1999, 8, 673–689. [Google Scholar] [CrossRef]
- Trivedi, N.; Hollister-Lock, J.; Lopez-Avalos, M.D.; O’Neil, J.J.; Keegan, M.; Bonner-Weir, S.; Weir, G.C. Increase in beta-cell mass in transplanted porcine neonatal pancreatic cell clusters is due to proliferation of beta-cells and differentiation of duct cells. Endocrinology 2001, 142, 2115–2122. [Google Scholar] [CrossRef]
- Lopez-Avalos, M.D.; Tatarkiewicz, K.; Sharma, A.; Bonner-Weir, S.; Weir, G.C. Enhanced maturation of porcine neonatal pancreatic cell clusters with growth factors fails to improve transplantation outcome. Transplantation 2001, 71, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.H.; Kuo, C.H.; Yao, N.K. Effects of insulin-like growth factor-1 and donor age on transplantation of porcine neonatal pancreatic cell clusters. Transplant. Proc. 2009, 41, 1794–1796. [Google Scholar] [CrossRef] [PubMed]
- Kin, T.; Korbutt, G.S.; Kobayashi, T.; Dufour, J.M.; Rajotte, R.V. Reversal of diabetes in pancreatectomized pigs after transplantation of neonatal porcine islets. Diabetes 2005, 54, 1032–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardona, K.; Korbutt, G.S.; Milas, Z.; Milas, Z.; Lyon, J.; Cano, J.; Jiang, W.; Bello-Laborn, H.; Hacquoil, B.; Strobert, E.; et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat. Med. 2006, 12, 304–306. [Google Scholar] [CrossRef]
- Li, W.C.; Chen, C.Y.; Kao, C.W.; Huang, P.C.; Hsieh, Y.T.; Kuo, T.Y.; Chen, T.Y.; Chia, H.Y.; Juang, J.H. Porcine Neonatal Pancreatic Cell Clusters Maintain Their Multipotency in Culture and After Transplantation. Sci. Rep. 2018, 8, 8212. [Google Scholar] [CrossRef] [PubMed]
- Evgenov, N.V.; Medarova, Z.; Dai, G.; Bonner-Weir, S.; Moore, A. In vivo imaging of islet transplantation. Nat. Med. 2006, 12, 144–148. [Google Scholar] [CrossRef] [Green Version]
- Evgenov, N.V.; Medarova, Z.; Pratt, J.; Pantazopoulos, P.; Leyting, S.; Bonner-Weir, S.; Moore, A. In vivo imaging of immune rejection in transplanted pancreatic islets. Diabetes 2006, 55, 2419–2428. [Google Scholar] [CrossRef] [Green Version]
- Evgenov, N.V.; Pratt, J.; Pantazopoulos, P.; Moore, A. Effects of glucose toxicity and islet purity on in vivo magnetic resonance imaging of transplanted pancreatic islets. Transplantation 2008, 85, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Hathout, E.; Chan, N.K.; Tan, A.; Sakata, N.; Mace, J.; Pearce, W.; Peverini, R.; Chinnock, R.; Sowers, L.; Obenaus, A. In vivo imaging demonstrates a time-line for new vessel formation in islet transplantation. Pediatr. Transplant. 2009, 13, 892–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jirák, D.; Kríz, J.; Herynek, V.; Andersson, B.; Girman, P.; Burian, M.; Saudek, F.; Hájek, M. MRI of transplanted pancreatic islets. Magn. Reson. Med. 2004, 52, 1228–1233. [Google Scholar] [CrossRef]
- Berkova, Z.; Kriz, J.; Girman, P.; Zacharovova, K.; Koblas, T.; Dovolilova, E.; Saudek, F. Vitality of pancreatic islets labeled for magnetic resonance imaging with iron particles. Transplant. Proc. 2005, 37, 3496. [Google Scholar] [CrossRef]
- Kriz, J.; Jirák, D.; Girman, P.; Berková, Z.; Zacharovova, K.; Honsova, E.; Lodererova, A.; Hajek, M.; Saudek, F. Magnetic resonance imaging of pancreatic islets in tolerance and rejection. Transplantation 2005, 80, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Tai, J.H.; Foster, P.; Rosales, A.; Moore, A. Imaging islets labeled with magnetic nanoparticles at 1.5 Tesla. Diabetes 2006, 55, 2931–2938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkova, Z.; Jirak, D.; Zacharovova, K.; Kriz, J.; Lodererova, A.; Girman, P.; Koblas, T.; Dovolilova, E.; Vancova, M.; Hajek, M.; et al. Labeling of pancreatic islets with iron oxide nanoparticles for in vivo detection with magnetic resonance. Transplantation 2008, 85, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Peng, Z.H.; Xing, T.H.; Qin, J.; Zhong, C.P. Assessment of islet graft survival using a 3.0-Tesla magnetic resonance scanner. Anat. Rec. 2008, 291, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; He, H.; Lu, W.; Xu, Q.; Zhou, B.; Tang, X. Tracking intrahepatically transplanted islets labeled with Feridex-polyethyleneimine complex using a clinical 3.0-T magnetic resonance imaging scanner. Pancreas 2009, 38, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Marzola, P.; Longoni, B.; Szilagyi, E.; Merigo, F.; Nicolato, E.; Fiorini, S.; Paoli, G.T.; Benati, D.; Mosca, F.; Sbarbati, A. In vivo visualization of transplanted pancreatic islets by MRI: Comparison between in vivo, histological and electron microscopy findings. Contrast Media Mol. Imaging 2009, 4, 135–142. [Google Scholar] [CrossRef]
- Medarova, Z.; Vallabhajosyula, P.; Tena, A.; Evgenov, N.; Pantazopoulos, P.; Tchipashvili, V.; Weir, G.; Sachs, D.; Moore, A. In vivo imaging of autologous islet grafts in the liver and under the kidney capsule in non-human primates. Transplantation 2009, 87, 1659–1666. [Google Scholar] [CrossRef]
- Toso, C.; Vallee, J.P.; Morel, P.; Ris, F.; Demuylder-Mischler, S.; Lepetit-Coiffe, M.; Marangon, N.; Saudek, F.; Shapiro, A.M.J.; Bosco, D.; et al. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am. J. Transplant. 2008, 8, 701–706. [Google Scholar] [CrossRef]
- Saudek, F.; Jirák, D.; Girman, P.; Herynek, V.; Dezortová, M.; Kríz, J.; Peregrin, J.; Berková, Z.; Zacharovová, K.; Hájek, M. Magnetic resonance imaging of pancreatic islets transplanted into the liver in humans. Transplantation 2010, 90, 1602–1606. [Google Scholar] [CrossRef]
- Cher, T.; Szklaruk, J. MR contrast agents: Applications in hepatobiliary imaging. Appl. Radiol. 2010, 39, 26–42. [Google Scholar]
- Kumar, M.N.; Muzzarelli, R.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Poon, Y.F.; Chan-Park, M.B.; Chen, Y.; Zhang, Q. Individually dispersing single-walled carbon nanotubes with novel neutral pH water-soluble chitosan derivatives. J. Phys. Chem. C 2008, 112, 7579–7587. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Wang, L.; Wang, A. Manipulated dispersion of carbon nanotubes with derivatives of chitosan. Carbon 2007, 45, 1917–1920. [Google Scholar] [CrossRef]
- Laudenslager, M.J.; Schiffman, J.D.; Schauer, C.L. Carboxymethyl chitosan as a matrix material for platinum, gold, and silver nanoparticles. Biomacromolecules 2008, 9, 2682–2685. [Google Scholar] [CrossRef]
- Klepka, M.T.; Nedelko, N.; Greneche, J.M.; Lawniczak-Jablonska, K.; Demchenko, I.N.; Rodrigues, C.A.; Debrassi, A.; Bordini, C. Local atomic structure and magnetic ordering of iron in Fe-chitosan complexes. Biomacromolecules 2008, 9, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Bhati, S.; Ravi, N. A Mössbauer study of the interaction of chitosan and D-glucosamine with iron and its relevance to other metalloenzymes. Biomacromolecules 2003, 4, 723–727. [Google Scholar] [CrossRef]
- Tsai, Z.-T.; Wang, J.-F.; Kuo, H.-Y.; Shen, C.-R.; Wang, J.-J.; Yen, T.-C. In situ preparation of high relaxivity iron oxide nanoparticles by coating with chitosan: A potential MRI contrast agent useful for cell tracking. J. Magn. Magn. Mater. 2010, 322, 208–213. [Google Scholar] [CrossRef]
- Shen, C.-R.; Juang, J.-H.; Tsai, Z.-T.; Wu, S.-T.; Tsai, F.-Y.; Wang, J.-J.; Liu, C.-L.; Yen, T.-C. Preparation, characterization and application of superparamagnetic iron oxide encapsulated with N-[(2-hydroxy-3-trimethylammonium) propyl] chitosan chloride. Carbohydr. Polym. 2011, 84, 781–787. [Google Scholar] [CrossRef]
- Juang, J.H.; Shen, C.R.; Wang, J.J.; Kuo, C.H.; Chien, Y.W.; Kuo, H.Y.; Chen, F.R.; Chen, M.H.; Yen, T.C.; Tsai, Z.T. Magnetic resonance imaging of mouse islet grafts labeled with novel chitosan-coated superparamagnetic iron oxide nanoparticles. PLoS ONE 2013, 8, e62626. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.H.; Wang, J.J.; Shen, C.R.; Kuo, C.H.; Chien, Y.W.; Kuo, H.Y.; Tsai, Z.T.; Yen, T.C. Magnetic resonance imaging of transplanted mouse islets labeled with chitosan-coated superparamagnetic iron oxide nanoparticles. Transplant. Proc. 2010, 42, 2104–2108. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.H.; Shen, C.R.; Wang, J.J.; Kuo, C.H.; Lin, M.Y.; Wu, S.T.; Tsai, Z.T.; Yen, T.C. Magnetic resonance imaging study of mouse islet allotransplantation. Transplant. Proc. 2010, 42, 4217–4220. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Ma, Z.; Khor, E.; Lim, L.Y. Uptake of FITC-chitosan nanoparticles by A549 cells. Pharm. Res. 2002, 19, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Wood, E.; Dornish, M. Effect of chitosan on epithelial cell tight junctions. Pharm Res 2004, 21, 43–49. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juang, J.-H.; Wang, J.-J.; Shen, C.-R.; Chen, C.-Y.; Kao, C.-W.; Chen, C.-L.; Lin, S.-H.; Wu, S.-T.; Li, W.-C.; Tsai, Z.-T. Magnetic Resonance Imaging of Transplanted Porcine Neonatal Pancreatic Cell Clusters Labeled with Chitosan-Coated Superparamagnetic Iron Oxide Nanoparticles in Mice. Polymers 2021, 13, 1238. https://doi.org/10.3390/polym13081238
Juang J-H, Wang J-J, Shen C-R, Chen C-Y, Kao C-W, Chen C-L, Lin S-H, Wu S-T, Li W-C, Tsai Z-T. Magnetic Resonance Imaging of Transplanted Porcine Neonatal Pancreatic Cell Clusters Labeled with Chitosan-Coated Superparamagnetic Iron Oxide Nanoparticles in Mice. Polymers. 2021; 13(8):1238. https://doi.org/10.3390/polym13081238
Chicago/Turabian StyleJuang, Jyuhn-Huarng, Jiun-Jie Wang, Chia-Rui Shen, Chen-Yi Chen, Chen-Wei Kao, Chen-Ling Chen, Sung-Han Lin, Shu-Ting Wu, Wan-Chun Li, and Zei-Tsan Tsai. 2021. "Magnetic Resonance Imaging of Transplanted Porcine Neonatal Pancreatic Cell Clusters Labeled with Chitosan-Coated Superparamagnetic Iron Oxide Nanoparticles in Mice" Polymers 13, no. 8: 1238. https://doi.org/10.3390/polym13081238