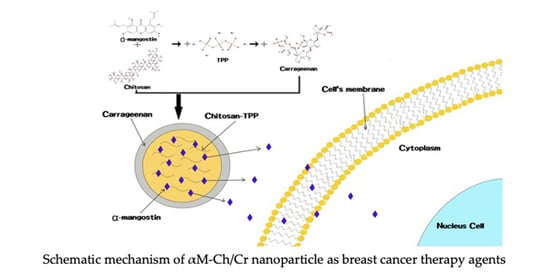
The Potential Cytotoxic Activity Enhancement of α-Mangostin in Chitosan-Kappa Carrageenan-Loaded Nanoparticle against MCF-7 Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of αM Chitosan Nanoparticles
2.3. Preparation of αM Chitosan-Loaded Kappa Carrageenan Nanoparticles
2.4. Scanning Electron Microscopy (SEM)
2.5. Entrapment Efficiency and Drug Loading
2.6. Fourier-Transform Infrared Spectroscopy (FTIR)
2.7. X-ray Diffraction (XRD)
2.8. Differential Scanning Calorimetry (DSC)
2.9. Saturation Solubility Study
2.10. In Vitro Drug Release
2.11. In Vitro Cytotoxicity
2.12. Statistical Analysis
3. Results
3.1. Characterization of Nanoparticles
3.1.1. SEM, Particle Size, Entrapment Efficiency, and Drug Loading
3.1.2. FTIR Analysis
3.1.3. XRD Analysis
3.1.4. DSC Analysis
3.2. In Vitro Studies
3.2.1. Saturation Solubility Study
3.2.2. In Vitro Release
3.2.3. In Vitro Cytotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nounou, M.I.; Elamrawy, F.; Ahmed, N.; Abdelraouf, K.; Goda, S.; Syed-Sha-Qhattal, H. Breast Cancer: Conventional Diagnosis and Treatment Modalities and Recent Patents and Technologies Supplementary Issue: Targeted Therapies in Breast Cancer Treatment. Breast Cancer Basic Clin. Res. 2015, 9, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Ganoth, A.; Merimi, K.C.; Peer, D. Overcoming Multidrug Resistance with Nanomedicines. Expert Opin. Drug Deliv. 2015, 12, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, F.; Volkov, N.; Bogdanov, A.; Dubina, M.; Moiseyenko, V. Resistance Mechanisms to Drug Therapy in Breast Cancer and Other Solid Tumors: An Opinion. F1000Research 2017, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Benjakul, R.; Kongkaneramit, L.; Sarisuta, N.; Moongkarndi, P.; Müller-Goymann, C.C. Cytotoxic Effect and Mechanism Inducing Cell Death of α-Mangostin Liposomes in Various Human Carcinoma and Normal Cells. Anti-Cancer Drugs 2015, 26, 824–834. [Google Scholar] [CrossRef]
- Kritsanawong, S.; Innajak, S.; Imoto, M.; Watanapokasin, R. Antiproliferative and Apoptosis Induction of α-Mangostin in T47D Breast Cancer Cells. Int. J. Oncol. 2016, 48, 2155–2165. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.K.; Yu, W.; Shrivastava, A.; Shankar, S.; Srivastava, R.K. α-Mangostin-Encapsulated PLGA Nanoparticles Inhibit Pancreatic Carcinogenesis by Targeting Cancer Stem Cells in Human, and Transgenic (KrasG12D, and KrasG12D/Tp53R270H) Mice. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Samprasit, W.; Akkaramongkolporn, P.; Jaewjira, S.; Opanasopit, P. Design of Alpha Mangostin-Loaded Chitosan/Alginate Controlled-Release Nanoparticles Using Genipin as Crosslinker. J. Drug Deliv. Sci. Technol. 2018, 46, 312–321. [Google Scholar] [CrossRef]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent Advances of Chitosan Nanoparticles as Drug Carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Pridgen, E.M.; Alexis, F.; Farokhzad, O.C. Polymeric Nanoparticle Technologies for Oral Drug Delivery. Clin. Gastroenterol. Hepatol. 2014, 12, 1605–1610. [Google Scholar] [CrossRef] [Green Version]
- Hassani, S.; Laouini, A.; Fessi, H.; Charcosset, C. Preparation of Chitosan-TPP Nanoparticles Using Microengineered Membranes-Effect of Parameters and Encapsulation of Tacrine. Colloids Surf. Physicochem. Eng. Asp. 2015, 482, 34–43. [Google Scholar] [CrossRef]
- Luppi, B.; Bigucci, F.; Abruzzo, A.; Corace, G.; Cerchiara, T.; Zecchi, V. Freeze-Dried Chitosan/Pectin Nasal Inserts for Antipsychotic Drug Delivery. Eur. J. Pharm. Biopharm. 2010, 75, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Samprasit, W.; Opanasopit, P. Chitosan-Based Nanoparticles for Controlled-Release Delivery of α–Mangostin. Int. J. Pharma Med. Biol. Sci. 2020, 9, 1–5. [Google Scholar] [CrossRef]
- Taher, F.A.; Ibrahim, S.A.; El-Aziz, A.A.; Abou El-Nour, M.F.; El-Sheikh, M.A.; El-Husseiny, N.; Mohamed, M.M. Anti-Proliferative Effect of Chitosan Nanoparticles (Extracted from Crayfish Procambarus Clarkii, Crustacea: Cambaridae) against MDA-MB-231 and SK-BR-3 Human Breast Cancer Cell Lines. Int. J. Biol. Macromol. 2019, 126, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Abedia, Z.; Moghadamnia, A.A.; Zabihi, E.; Pourbagher, R.; Ghasemi, M.; Nouri, H.R.; Tashakorian, H.; Jenabian, N. Anticancer Properties of Chitosan against Osteosarcoma, Breast Cancer and Cervical Cancer Cell Lines. Casp. J. Intern. Med. 2019, 10, 439–446. [Google Scholar] [CrossRef]
- Jiang, Z.; Han, B.; Li, H.; Yang, Y.; Liu, W. Carboxymethyl Chitosan Represses Tumor Angiogenesis in Vitro and in Vivo. Carbohydr. Polym. 2015, 129, 1–8. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Gavini, E.; Rassu, G.; Maestri, M.; Giunchedi, P. Chitosan Nanoparticles for Therapy and Theranostics of Hepatocellular Carcinoma (HCC) and Liver-Targeting. Nanomaterials 2020, 10, 870. [Google Scholar] [CrossRef]
- Ai, J.W.; Liao, W.; Ren, Z.L. Enhanced Anticancer Effect of Copper-Loaded Chitosan Nanoparticles against Osteosarcoma. RSC Adv. 2017, 7, 15971–15977. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, H.S.; Yadav, P.N. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int. J. Biomater. 2018, 2018, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Vivek, R.; Nipun Babu, V.; Thangam, R.; Subramanian, K.S.; Kannan, S. PH-Responsive Drug Delivery of Chitosan Nanoparticles as Tamoxifen Carriers for Effective Anti-Tumor Activity in Breast Cancer Cells. Colloids Surf. B Biointerfaces 2013, 111, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, M.A.; Madni, A.; Rehman, M.; Rahim, M.A.; Jabar, A. Ionically Cross-Linked Chitosan Nanoparticles for Sustained Delivery of Docetaxel: Fabrication, Post-Formulation and Acute Oral Toxicity Evaluation. Int. J. Nanomed. 2019, 14, 10035–10046. [Google Scholar] [CrossRef] [Green Version]
- Jonassen, H.; Kjoniksen, A.L.; Hiorth, M. Stability of Chitosan Nanoparticles Cross-Linked with Tripolyphosphate. Biomacromolecules 2012, 13, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Winnicka, K. Stability of Chitosan-A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and Its Applications in Drug Delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.V.; Sato, T. Encapsulation of Glucose Oxidase (GOD) in Polyelectrolyte Complexes of Chitosan-Carrageenan. React. Funct. Polym. 2010, 70, 19–27. [Google Scholar] [CrossRef]
- Bulmer, C.; Margaritis, A.; Xenocostas, A. Encapsulation and Controlled Release of Recombinant Human Erythropoietin from Chitosan-Carrageenan Nanoparticles. Curr. Drug Deliv. 2012, 9, 527–537. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Karimi, M.H.; Soltaniniya, M.; Massoumi, B. In Vitro Evaluation of Sustained Ciprofloxacin Release from κ-Carrageenan-Crosslinked Chitosan/Hydroxyapatite Hydrogel Nanocomposites. Int. J. Biol. Macromol. 2019, 126, 443–453. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Zhao, Z.; Li, J.; Zhang, R.; Yao, F. Formation and Characterization of Natural Polysaccharide Hollow Nanocapsules via Template Layer-by-Layer Self-Assembly. J. Colloid Interface Sci. 2012, 379, 130–140. [Google Scholar] [CrossRef]
- Wathoni, N.; Rusdin, A.; Febriani, E.; Purnama, D.; Daulay, W.; Azhary, S.Y.; Panatarani, C.; Joni, I.M.; Lesmana, R.; Keiichi, M.; et al. Formulation and Characterization of α-Mangostin in Chitosan Nanoparticles Coated by Sodium Alginate, Sodium Silicate, and Polyethylene Glycol. J. Pharm. Bioallied Sci. 2019, 20, 1–9. [Google Scholar] [CrossRef]
- Smith, B. Fundamentals of Fourier Transform Infrared Spectroscopy, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar] [CrossRef]
- Zak, A.; Majid, W.; ME, A.; Yousefi, R. X-Ray Analysis of ZnO Nanoparticles by Williamson–Hall and Size–Strain Plot Methods. Solid State Sci. 2011, 13, 251. [Google Scholar] [CrossRef]
- Sarmento, B.; Ferreira, D.; Veiga, F.; Ribeiro, A. Characterizationof Insulin-Loaded Alginate Nanoparticles Produced Byionotropic Pre-Gelation through DSC and FTIR Studies. Carbohydr Polym. 2006, 66, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Baka, E.; Comer, J.E.A.; Takács-Novák, K. Study of Equilibrium Solubility Measurement by Saturation Shake-Flask Method Using Hydrochlorothiazide as Model Compound. J. Pharm. Biomed. Anal. 2008, 46, 335–341. [Google Scholar] [CrossRef]
- Singhvi, G.; Singh, M. In-Vitro Drug Release Characterization Models. Int. J. Pharm. Stud. Res. 2011, 2, 77–84. [Google Scholar]
- CCRC, U. Standard Procedure of the Cytotoxic Test MTT Method. Cancer Chemoprevention Res. Cent. 2012, 2, 1–7. [Google Scholar]
- Nelli, G.B.; Anand Solomon, K.; Kilari, E.K. Antidiabetic Effect of α-Mangostin and Its Protective Role in Sexual Dysfunction of Streptozotocin Induced Diabetic Male Rats. Syst. Biol. Reprod. Med. 2013, 59, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, S.; Kabiraz, M.; Saha, B.; Qadir, M.; Gafur, M.; Masum, S. Chromium (VI) Ions Removal from Tannery Effluent Using Chitosan-Microcrystalline Cellulose Composite as Adsorbent. Int. Res. J. Pure Appl. Chem. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Loutfy, S.A.; Salaheldin, T.A.; Ramadan, M.A.; Farroh, K.Y.; Abdallah, Z.F.; Eloahed, T.Y.A. Synthesis, Characterization and Cytotoxic Evaluation of Graphene Oxide Nanosheets: In Vitro Liver Cancer Model. Asian Pac. J. Cancer Prev. 2017, 18, 955–961. [Google Scholar] [CrossRef]
- Mahmood, W.A.K.; Khan, M.M.R.; Yee, T.C. Effects of Reaction Temperature on the Synthesis and Thermal Properties of Carrageenan Ester. J. Phys. Sci. 2014, 25, 123–138. [Google Scholar]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Reineck, P.; Brick, D.; Mulvaney, P.; Bach, U. Plasmonic Hot Electron Solar Cells: The Effect of Nanoparticle Size on Quantum Efficiency. J. Phys. Chem. Lett. 2016, 7, 4137–4141. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; Eastoe, J.; Lord, A.M.; Guittard, F.; Barron, A.R. Branched Hydrocarbon Low Surface Energy Materials for Superhydrophobic Nanoparticle Derived Surfaces. ACS Appl. Mater. Interfaces 2015, 8, 660–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arvaniti, E.C.; Juenger, M.C.G.; Bernal, S.A.; Duchesne, J.; Courard, L.; Leroy, S.; Provis, J.L.; Klemm, A.; De Belie, N. Determination of Particle Size, Surface Area, and Shape of Supplementary Cementitious Materials by Different Techniques. Mater. Struct. 2015, 48, 3687–3701. [Google Scholar] [CrossRef] [Green Version]
- Li, C.F.; Li, Y.C.; Chen, L.B.; Wang, Y.; Sun, L.B. Doxorubicin-Loaded Eudragit-Coated Chitosan Nanoparticles in the Treatment of Colon Cancers. J. Nanosci. Nanotechnol. 2016, 16, 6773–6780. [Google Scholar] [CrossRef]
- Acosta-Ferreira, S.; Castillo, O.S.; Madera-Santana, J.T.; Mendoza-García, D.A.; Núñez-Colín, C.A.; Grijalva-Verdugo, C.; Villa-Lerma, A.G.; Morales-Vargas, A.T.; Rodríguez-Núñez, J.R. Production and Physicochemical Characterization of Chitosan for the Harvesting of Wild Microalgae Consortia. Biotechnol. Rep. 2020, 28. [Google Scholar] [CrossRef]
- Dickmann, R.S.; Strasburg, G.M.; Romsos, D.R.; Wilson, L.A.; Lai, G.H.; Huang, H. Particle Size, Surface Area, and Amorphous Content as Predictors of Solubility and Bioavailability for Five Commercial Sources of Ferric Orthophosphate in Ready-to-Eat Cereal. Nutrients 2016, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Qian, S.; Heng, W.; Wei, Y.; Zhang, J.; Gao, Y. Coamorphous Lurasidone Hydrochloride-Saccharin with Charge-Assisted Hydrogen Bonding Interaction Shows Improved Physical Stability and Enhanced Dissolution with Ph-Independent Solubility Behavior. Cryst. Growth Des. 2015, 15, 2920–2928. [Google Scholar] [CrossRef]
- Wlodarski, K.; Sawicki, W.; Haber, K.; Knapik, J.; Wojnarowska, Z.; Paluch, M.; Lepek, P.; Hawelek, L.; Tajber, L. Physicochemical Properties of Tadalafil Solid Dispersions-Impact of Polymer on the Apparent Solubility and Dissolution Rate of Tadalafil. Eur. J. Pharm. Biopharm. 2015, 94, 106–115. [Google Scholar] [CrossRef]
- Grenha, A.; Gomes, M.E.; Rodrigues, M.; Santo, V.E.; Mano, J.F.; Neves, N.M.; Reis, R.L. Development of New Chitosan/Carrageenan Nanoparticles for Drug Delivery Applications. J. Biomed. Mater. Res. Part J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 92, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Du, Y. Effect of Molecular Structure of Chitosan on Protein Delivery Properties of Chitosan Nanoparticles. Int. J. Pharm. 2003, 250, 215–226. [Google Scholar] [CrossRef]
- Nabekura, T.; Yamaki, T.; Hiroi, T.; Ueno, K.; Kitagawa, S. Inhibition of Anticancer Drug Efflux Transporter P-Glycoprotein by Rosemary Phytochemicals. Pharmacol. Res. 2010, 61, 259–263. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tsujiuchi, T.; Okui, Y.; Mizutani, A.; Nishi, K.; Nakanishi, T.; Nishii, R.; Fukuchi, K.; Tamai, I.; Kawai, K. Different Efflux Transporter Affinity and Metabolism of 99mTc-2-Methoxyisobutylisonitrile and 99mTc-Tetrofosmin for Multidrug Resistance Monitoring in Cancer. Pharm. Res. 2019, 36, 18. [Google Scholar] [CrossRef] [PubMed]
- Groult, H.; Cousin, R.; Chot-Plassot, C.; Maura, M.; Bridiau, N.; Piot, J.M.; Maugard, T.; Fruitier-Arnaudin, I. Λ-Carrageenan Oligosaccharides of Distinct Anti-Heparanase and Anticoagulant Activities Inhibit MDA-MB-231 Breast Cancer Cell Migration. Mar. Drugs 2019, 17, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasedya, E.S.; Miyake, M.; Kobayashi, D.; Hazama, A. Carrageenan Delays Cell Cycle Progression in Human Cancer Cells in Vitro Demonstrated by FUCCI Imaging. BMC Complement. Altern. Med. 2016, 16, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murad, H.; Ghannam, A.; Al-Ktaifani, M.; Abbas, A.; Hawat, M. Algal Sulfated Carrageenan Inhibits Proliferation of MDA-MB-231 Cells via Apoptosis Regulatory Genes. Mol. Med. Rep. 2015, 11, 2153–2158. [Google Scholar] [CrossRef] [PubMed]






| Formulation | F1 | F2 | F3 |
|---|---|---|---|
| αM (mg) | 20 | 20 | 20 |
| Chitosan (mg) | 200 | 200 | 200 |
| Sodium tripolyphosphate (mg) | 40 | 40 | 40 |
| Kappa carrageenan (mg) | 25 | 45 | 85 |
| Formulae | Entrapment Efficiency (%) | Drug Loading (%) |
|---|---|---|
| F1 | 98.74 ± 1.20 | 6.93 ± 0.87 |
| F2 | 99.35 ± 1.10 | 6.51 ± 0.86 |
| F3 | 98.59 ± 0.98 | 5.72 ± 0.60 |
| Material | Wavenumber (cm−1) | Functional Groups | Reference | |
|---|---|---|---|---|
| Result | Literature | |||
| α-mangostin | 34,081; 32,204 | 3260 | O–H stretch | [37] |
| 29,875; 26,078; 29,317 | 2989; 2962; 2924 | C–H stretch | ||
| 16,338 | 1642 | C=O | ||
| 14,435 | 1454 | C–C | ||
| 11,782 | 1199 | Ortho–OCH3 stretch | ||
| 107,533 | 1076 | C–O–C stretch | ||
| Chitosan | 350,954 | 347,868 | O–H stretch dan N–H stretch | [38] |
| 289,424 | 292,413 | C–H stretch | ||
| 165,302 | 165,688 | C=O | ||
| 1597 | 157,105 | N–H bend | ||
| 141,963 | 142,253 | C–H bend | ||
| 137,816 | 137,816 | C–N | ||
| 115,731 | 115,731 | C–O–C stretch | ||
| 1079 | 102,518 | C–O | ||
| Sodium tripolyphosphate | 121,132 | 1210 | νas P=O | [39] |
| 115,731 | 1130 | νs O–P=O | ||
| 109,269 | 1090 | νas PO3 | ||
| 88,823 | 888 | νas P–O–P | ||
| Kappa carrageenan | 1262 | 1241 | O=S=O | |
| 1069 | 1069 | Glycosidic bond | ||
| 929 | 922 | 3,6-anhydrogalactose | [40] | |
| 846 | 847 | Galactose-4-sulfate | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wathoni, N.; Meylina, L.; Rusdin, A.; Mohammed, A.F.A.; Tirtamie, D.; Herdiana, Y.; Motoyama, K.; Panatarani, C.; Joni, I.M.; Lesmana, R.; et al. The Potential Cytotoxic Activity Enhancement of α-Mangostin in Chitosan-Kappa Carrageenan-Loaded Nanoparticle against MCF-7 Cell Line. Polymers 2021, 13, 1681. https://doi.org/10.3390/polym13111681
Wathoni N, Meylina L, Rusdin A, Mohammed AFA, Tirtamie D, Herdiana Y, Motoyama K, Panatarani C, Joni IM, Lesmana R, et al. The Potential Cytotoxic Activity Enhancement of α-Mangostin in Chitosan-Kappa Carrageenan-Loaded Nanoparticle against MCF-7 Cell Line. Polymers. 2021; 13(11):1681. https://doi.org/10.3390/polym13111681
Chicago/Turabian StyleWathoni, Nasrul, Lisna Meylina, Agus Rusdin, Ahmed Fouad Abdelwahab Mohammed, Dorandani Tirtamie, Yedi Herdiana, Keiichi Motoyama, Camelia Panatarani, I Made Joni, Ronny Lesmana, and et al. 2021. "The Potential Cytotoxic Activity Enhancement of α-Mangostin in Chitosan-Kappa Carrageenan-Loaded Nanoparticle against MCF-7 Cell Line" Polymers 13, no. 11: 1681. https://doi.org/10.3390/polym13111681