Fabrication and Characterization of Chitosan/Cellulose Nanocrystal/Glycerol Bio-Composite Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of CNC
2.3. Preparation of Chitosan/CNC/Glycerol Bio-Composite Films
2.4. X-ray Diffraction (XRD) Analysis
2.5. Fourier Transform Infrared (FT-IR) Spectra Analysis
2.6. Thermal Stability
2.7. Tensile Properties
2.8. Water Absorption
2.9. Light Transmittance Analysis
3. Results and Discussion
3.1. XRD Analysis
3.2. FT-IR Analysis
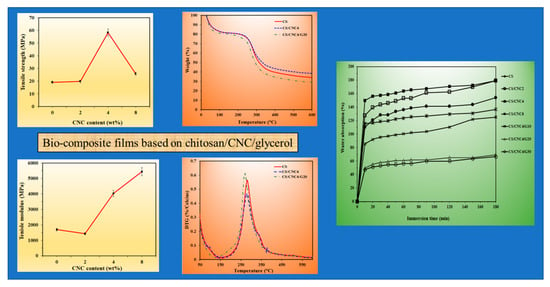
3.3. Thermal Stability
3.4. Tensile Properties
3.5. Water Absorption
3.6. Light Transmittance Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García, N.L.; Ribba, L.; Dufresne, A.; Aranguren, M.; Goyanes, S. Effect of glycerol on the morphology of nanocomposites made from thermoplastic starch and starch nanocrystals. Carbohydr. Polym. 2011, 84, 203–210. [Google Scholar] [CrossRef]
- Holcapkova, P.; Stloukal, P.; Kucharczyk, P.; Omastova, M.; Kovalcik, A. Anti-hydrolysis effect of aromatic carbodiimide in poly (lactic acid)/wood flour composites. Compos. Part A 2017, 103, 283–291. [Google Scholar] [CrossRef]
- Zactiti, E.M.; Kieckbusch, T.G. Release of potassium sorbate from active films of sodium alginate crosslinked with calcium chloride. Packag. Technol. Sci. 2009, 22, 349–358. [Google Scholar] [CrossRef]
- Norajit, K.; Kim, K.M.; Ryu, G.H. Comparative studies on the characterization and antioxidant properties of biodegradable alginate films containing ginseng extract. J. Food Eng. 2010, 98, 377–384. [Google Scholar] [CrossRef]
- Atef, M.; Rezaei, M.; Behrooz, R. Preparation and characterization agar-based nanocomposite film reinforced by nanocrystalline cellulose. Int. J. Biol. Macromol. 2014, 70, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhao, X.; Wang, D.; Dong, J.; She, D.; Peng, P. Preparation and characterization of nanocrystalline cellulose/Eucommia ulmoides gum nanocomposite film. Carbohydr. Polym. 2018, 181, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Manrich, A.; Moreira, F.K.; Otoni, C.G.; Lorevice, M.V.; Martins, M.A.; Mattoso, L.H. Hydrophobic edible films made up of tomato cutin and pectin. Carbohydr. Polym. 2017, 164, 83–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Khan, R.A.; Salmieri, S.; Tien, C.L.; Riedl, B.; Bouchard, J.; Chauve, G.; Tan, V.; Kamal, M.R.; Lacroix, M. Mechanical and barrier properties of cellulose nanocrystal reinforced chitosan-based nanocomposite films. Carbohydr. Polym. 2012, 90, 1601–1608. [Google Scholar] [CrossRef]
- Celebi, H.; Kurt, A. Effects of processing on the properties of chitosan/cellulose nanocrsytal films. Carbohydr. Polym. 2015, 133, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Abdurrahim, I. Water sorption, antimicrobial activity, and thermal and mechanical properties of chitosan/clay/glycerol nanocomposite films. Heliyon 2019, 5, e02342. [Google Scholar]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Development and characterization of sugar palm cellulose nanocrystalreinforced sugar palm starch bionanocomposites. Carbohydr. Polym. 2018, 202, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Aryaei, A.; Jayatissaa, A.H.; Jayasuriya, A.C. Mechanical and biological properties of chitosan/carbon nanotube nanocomposite films. J. Biomed. Mater. Res. A 2014, 102, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Corsello, F.A.; Bolla, P.A.; Anbinder, P.S.; Serradell, M.A.; Amalvy, J.I.; Peruzzo, P.J. Morphology and properties of neutralized chitosan-cellulose nanocrystals biocomposite films. Carbohydr. Polym. 2017, 156, 452–459. [Google Scholar] [CrossRef] [PubMed]
- De Mesquita, J.P.; Donnici, C.L.; Pereira, F.V. Biobased nanocomposites from layer-by-layer assembly of cellulose nanowhiskers with chitosan. Biomacromolecules 2010, 11, 473–480. [Google Scholar] [CrossRef]
- Li, H.Z.; Chen, S.C.; Wang, Y.Z. Preparation and characterization of nanocomposites of polyvinyl alcohol/cellulose nanowhiskers/chitosan. Compos. Sci. Technol. 2015, 115, 60–65. [Google Scholar] [CrossRef]
- Falamrzpour, P.; Behzad, T.; Zamani, A. Preparation of nanocellulose reinforced chitosan films, cross-linked by adipic acid. Int. J. Mol. Sci. 2017, 18, 396. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Liu, C.; Kang, K.; Zheng, Z.; Wang, S.; Tang, Z.; Yang, W. Isolation of cellulose nanocrystal from rice straw and preparation of its biocomposites with chitosan: Physicochemical, characterization and evaluation of interfacial compatibility. Compos. Sci. Technol. 2018, 154, 8–17. [Google Scholar] [CrossRef]
- Listyanda, R.F.; Kusmono; Wildan, M.W.; Ilman, M.N. Extraction and characterization of cellulose nanocrystal (CNC) from ramie fiber by sulphuric acid hydrolysis. AIP Conf. Proc. 2020, 2217, 030069. [Google Scholar]
- Tang, Y.; Zhang, X.; Zhao, R.; Guo, D.; Zhang, J. Preparation and properties of chitosan/guar gum/nanocrystalline cellulose nanocomposite films. Carbohydr. Polym. 2018, 197, 128–136. [Google Scholar] [CrossRef]
- Wang, S.F.; Shen, L.; Tong, Y.J.; Chen, L.; Phang, I.Y.; Lim, P.Q.; Liu, T.X. Biopolymer chitosan/montmorillonite nanocomposites: Preparation and characterization. Polym. Degrad. Stab. 2005, 90, 123–131. [Google Scholar] [CrossRef]
- Helbert, W.; Chanzy, H. Oriented growth of V amylase n-butanol crystals on cellulose. Carbohydr. Polym. 1994, 24, 119–122. [Google Scholar] [CrossRef]
- Gray, D.G. Transcrystallization of polypropylene at cellulose nanocrystal surfaces. Cellulose 2007, 15, 297–301. [Google Scholar] [CrossRef]
- Cosenza, L.W.; Bringaud, F.; Baltz, T.; Vellieux, F.M.D. Crystallization and preliminary crystallographic investigation of glycosomal pyruvate phosphate dikinase from Trypanosoma brucei. Acta Crystallogr. Sect. D Biol. Crystallogr. 2000, 56, 1688–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sit, N.; Misra, S.; Deka, S.C. Physicochemical, functional, textural and colour characteristics of starches isolated from four taro cultivars of North-East India. Starch-Stärke 2013, 65, 1011–1021. [Google Scholar] [CrossRef]
- Merino, D.; Mansilla, A.Y.; Gutiérrez, T.J.; Casalongué, C.A.; Alvarez, V.A. Chitosan coated-phosphorylated starch films: Water interaction, transparency and antibacterial properties. React. Funct. Polym. 2018, 131, 445–453. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, J.; Zhang, L. Structure and properties of the nanocomposite films of chitosan reinforced with cellulose whiskers. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 1069–1077. [Google Scholar] [CrossRef]
- Li, Q.; Renneckar, S. Supramolecular structure characterization of molecularly thin cellulose I nanoparticles. Biomacromolecules 2011, 12, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.; Salmieri, S.; Dussault, D.; Calderon, J.U.; Kamal, M.R.; Safrany, A.; Lacroix, M. Production and properties of nanocellulose reinforced methylcellulose based biodegradable films. J. Agric. Food Chem. 2010, 58, 7878–7885. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, A.; Mucha, M. Thermogravimetric and FTIR studies of chitosan blends. Thermochim. Acta 2003, 396, 153–166. [Google Scholar] [CrossRef]
- Martins, J.T.; Bourbon, A.I.; Pinheiro, A.C.; Souza, B.W.; Cerqueira, M.A.; Vicente, A.A. Biocomposite films based on-carrageenan/locust beangum blends and clays: Physical and antimicrobial properties. Food Bioprocess Technol. 2013, 6, 2081–2092. [Google Scholar] [CrossRef] [Green Version]
- Olivia, V.; López, O.V.; Ninago, M.D.; Soledad Lencina, M.M.; García, M.A.; Andreucetti, N.A.; Ciolino, A.E.; Villara, M.A. Thermoplastic starch plasticized with alginate–glycerol mixtures: Melt-processing evaluation and film properties. Carbohydr. Polym. 2015, 126, 83–90. [Google Scholar]
- Qi, G.; Li, N.; Sun, X.S.; Shi, Y.C.; Wang, D. Effects of glycerol and nanoclay on physiochemical properties of camelina gum-based films. Carbohydr. Polym. 2016, 152, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Cazón, P.; Velazquez, G.; Vázquez, M. Characterization of mechanical and barrier properties of bacterial cellulose, glycerol, and polyvinyl alcohol (PVOH) composite films with eco-friendly UV-protective properties. Food Hydrocoll. 2020, 99, 105323. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Enríquez-Ramírez, K.E.; García-Marquez, E.; Ramírez-Santiago, C.; Lobato-Calleros, C.; Vernon-Carter, J. Interrelationship between the zeta potential and viscoelastic properties in coacervates complexes. Carbohyd. Polym. 2013, 95, 161–166. [Google Scholar] [CrossRef]
- Heidarian, P.; Behzad, T.; Karimi, K.; Sain, M. Properties investigation of recycled polylactic acid reinforced by cellulose nanofibrils isolated from bagasse. Polym. Compos. 2018, 39, 740–3749. [Google Scholar] [CrossRef]
- Agustin, M.B.; Ahmmad, B.; Deleon, E.R.P.; Buenaobra, J.L.; Salazar, J.R.; Hirose, F. Starch-based biocomposite films reinforced with cellulose nanocrystals from garlic stalks. Polym. Compos. 2013, 34, 1325–1332. [Google Scholar] [CrossRef]
- Mangaraj, S.; Goswami, T.K.; Mahajan, P.V. Applications of plastic films for modified atmosphere packaging of fruits and vegetables: A review. Food Eng. Rev. 2009, 1, 133–158. [Google Scholar] [CrossRef]
- Sanyang, M.; Sapuan, S.M.; Jawaid, M.; Ishak, M.; Sshari, J. Effect of plasticizer type and concentration on tensile, thermal and barrier properties of biodegradable films based on sugar palm (Arenga pinnata) starch. Polymers 2015, 7, 1106–1124. [Google Scholar] [CrossRef]
- Hejazi, M.; Behzad, T.; Heidarian, P.; Nasri-Nasrabadi, B. A study of the effects of acid, plasticizer, cross-linker, and extracted chitin nanofibers on the properties of chitosan biofilm. Compos. Part A 2018, 109, 221–231. [Google Scholar] [CrossRef]
- Abral, H.; Basri, A.; Muhammad, F.; Fernando, Y.; Hafizulhaq, F.; Mahardika, M.; Sugiarti, E.; Sapuan, S.M.; Ilyas, R.A.; Stephaned, I. A simple method for improving the properties of the sago starch films prepared by using ultrasonication treatment. Food Hydrocoll. 2019, 93, 276–283. [Google Scholar] [CrossRef]
- Lavorgna, M.; Piscitelli, F.; Mangiacapra, P.; Buonocore, G.G. Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosan films. Carbohyd. Polym. 2010, 82, 291–298. [Google Scholar] [CrossRef]
- Tonny, W.; Tuhin, M.O.; Islam, R.; Khan, R.A. Fabrication and characterization of biodegradable packaging films using starch and chitosan: Effect of glycerol. J. Chem Eng. Chem. Res. 2014, 1, 343–352. [Google Scholar]
- Vlacha, M.; Giannakas, A.; Katapodis, P.; Stamatis, H.; Ladavos, A.; Barkoula, N.M. On the efficiency of oleic acid as plasticizer of chitosan/claynanocomposites and its role on thermo-mechanical, barrier and antimicrobial properties-Comparison with glycerol. Food Hydrocoll. 2016, 57, 10–19. [Google Scholar] [CrossRef]
- Tibolla, H.; Czaikoski, A.; Pelissari, F.M.; Menegalli, F.C.; Cunha, R.L. Starch-based nanocomposites with cellulose nanofibers obtained from chemical and mechanical treatments. Int. J. Biol. Macromol. 2020, 161, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Abral, H.; Kadriadi; Mahardika, M.; Handayani, D.; Sugiarti, E.; Muslimin, A.N. Characterization of disintegrated bacterial cellulose nanofibers/PVA bionanocomposites prepared via ultrasonication. Int. J. Biol. Macromol. 2019, 135, 591–599. [Google Scholar]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Sapuan, S.M.; Ilyas, R.A. Highly transparent and antimicrobial PVA based bionanocomposites reinforced by ginger nanofiber. Polym. Test. 2020, 81, 106186. [Google Scholar] [CrossRef]
- Abdollahi, M.; Alboofetileh, M.; Rezaei, M.; Behrooz, R. Comparing physico-mechanical and thermal properties of alginate nanocomposite films reinforced with organic and/or inorganic nanofillers. Food Hydrocoll. 2013, 32, 416–424. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Shaw, N.; Monahan, F.; O’Riordan, E.; O’sullivan, M. Effect of soya oil and glycerol on physical properties of composite WPI films. J. Food. Eng. 2002, 51, 299–304. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusmono; Wildan, M.W.; Lubis, F.I. Fabrication and Characterization of Chitosan/Cellulose Nanocrystal/Glycerol Bio-Composite Films. Polymers 2021, 13, 1096. https://doi.org/10.3390/polym13071096
Kusmono, Wildan MW, Lubis FI. Fabrication and Characterization of Chitosan/Cellulose Nanocrystal/Glycerol Bio-Composite Films. Polymers. 2021; 13(7):1096. https://doi.org/10.3390/polym13071096
Chicago/Turabian StyleKusmono, Muhammad Waziz Wildan, and Fadhlan Ihsan Lubis. 2021. "Fabrication and Characterization of Chitosan/Cellulose Nanocrystal/Glycerol Bio-Composite Films" Polymers 13, no. 7: 1096. https://doi.org/10.3390/polym13071096