Enhancement of Chloroprene/Natural/Butadiene Rubber Nanocomposite Properties Using Organoclays and Their Combination with Carbon Black as Fillers
Abstract
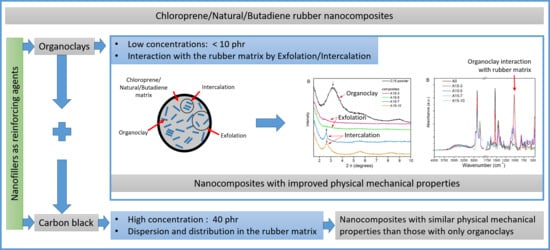
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Compounding and Vulcanization of Samples
2.3. Characterization Techniques
2.3.1. Physicomechanical Properties
2.3.2. X-Ray Diffraction Analysis
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Fourier Transformed Infrared (FT-IR) Analysis
2.3.5. Cure Characteristics
3. Results and Discussion
3.1. Physicomechanical Properties
3.1.1. Tear Properties
3.1.2. Tensile Properties
3.1.3. Abrasion Resistance
3.1.4. Hardness and Density
3.2. X-Ray Diffraction Analysis
3.3. Thermogravimetric Analysis
3.4. FT-IR Analysis
3.5. Cure Characteristics
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kapgate, B.P.; Das, C.; Das, A.; Basu, D.; Wiessner, S.; Reuter, U. Reinforced chloroprene rubber by in situ generated silica particles: Evidence of bound rubber on the silica surface. J. Appl. Polym. Sci. 2016, 43717, 1–10. [Google Scholar] [CrossRef]
- Wu, W.L.; Chen, Z. Modified-diatomite reinforced rubbers. Mater. Lett. 2017, 209, 159–162. [Google Scholar] [CrossRef]
- Garing, C.L.; Pajarito, B.B. Effect of clay loading on the water resistance of ternary-filled natural rubber composites. Mater. Today Proc. 2020, 33, 1959–1962. [Google Scholar] [CrossRef]
- Sethuramalingam, V.C.; Prabagaran, S.; Ganesan, K. Studies on influence of silica filler and rice husk ash on the mechanical properties of vulcanized hybrid rubber composite. Mater. Today Proc. 2020; in press. [Google Scholar] [CrossRef]
- Qiao, J. Elastomeric nano-particle and its applications in polymer modifications. Adv. Ind. Eng. Polym. Res. 2020, 3, 47–59. [Google Scholar] [CrossRef]
- Rajasekar, R.; Pal, K.; Heinrich, G.; Das, A.; Das, C.K. Development of nitrile butadiene rubber-nanoclay composites with epoxidized natural rubber as compatibilizer. Mater. Des. 2009, 30, 3839–3845. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Y.; Zhang, Y. Characterization of kaolinite/emulsion-polymerization styrene butadiene rubber (ESBR) nanocomposite prepared by latex blending method: Dynamic mechanic properties and mechanism. Polym. Test. 2020, 89, 1–11. [Google Scholar] [CrossRef]
- Ramesan, M.T.; Alex, R.; Khanh, N.V. Studies on the cure and mechanical properties of blends of natural rubber with dichlorocarbene modified styrene-butadiene rubber and chloroprene rubber. React. Funct. Polym. 2005, 62, 41–50. [Google Scholar] [CrossRef]
- Sirisinha, C.; Baulek-Limcharoen, S.; Thunyarittikorn, J. Relationships among blending conditions, size of dispersed phase, and oil resistance in natural rubber and nitrile rubber blends. J. Appl. Polym. Sci. 2001, 82, 1232–1237. [Google Scholar] [CrossRef]
- Choi, S.S. Improvement of properties of silica-filled natural rubber compounds using polychloroprene. J. Appl. Polym. Sci. 2002, 83, 2609–2616. [Google Scholar] [CrossRef]
- Razavi-Nouri, M.; Sabet, A.; Mohebbi, M. Thermal, tensile and rheological properties of dynamically cross-linked organoclay filled poly(ethylene-co-vinyl acetate)/acrylonitrile-butadiene rubber nanocomposites: Effect of peroxide content. Polymer 2020, 190, 1–10. [Google Scholar] [CrossRef]
- Kanny, K.; Mohan, T.P. Rubber nanocomposites with nanoclay as the filler. In Progress in Rubber Nanocomposites; Sabu, T., Hann, J.M., Eds.; Woodhead Publising: Durban, South Africa, 2017; pp. 153–177. ISBN 978-0081004098. [Google Scholar]
- Ahmed, K. An investigation on chloroprene-compatibilized acrylonitrile butadiene rubber/high density polyethylene blends. J. Adv. Res. 2014, 6, 811–817. [Google Scholar] [CrossRef] [Green Version]
- Manohar, N.; Jayaramudu, J.; Suchismita, S.; Rajkumar, K.; Badul Reddy, A.; Sadiku, E.R.; Priti, R.; Maurya, D.J. A unique application of the second order derivative of FTIR–ATR spectra for compositional analyses of natural rubber and polychloroprene rubber and their blends. Polym. Test. 2017, 62, 447–453. [Google Scholar] [CrossRef]
- Sare, I.R.; Mardel, J.I.; Hill, A.J. Wear-resistant metallic and elastomeric materials in the mining and mineral processing industries—An overview. Wear. 2001, 250, 1–10. [Google Scholar] [CrossRef]
- Lai Lai, P.L.; Joon Ching, J.; Nay Ming, H.; Leng Kian, G.; Fook Peng, L.; Yi Yee, L. Effect of graphene oxide particle size on the tensile strength and stability of natural rubber graphene composite. Mater. Sci. Eng. B 2020, 262, 1–10. [Google Scholar] [CrossRef]
- Senthilvel, K.; Arul Jeya Kumar, A.; Seeman, M.; Ashok Kumar, I.; Prabu, B. Studies the effect of halloysite nanotubes on the mechanical and hot air ageing properties of nitrile-polyvinyl chloride rubber nano-composites. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Rooj, S.; Das, A.; Stöckelhuber, K.W.; Wießner, S.; Fischer, D.; Reuter, U.; Heinrich, G. Expanded organoclay assisted dispersion and simultaneous structural alterations of multiwall carbon nanotube (MWCNT) clusters in natural rubber. Compos. Sci. Technol. 2015, 107, 36–43. [Google Scholar] [CrossRef]
- Zongchao, X.; Long, Z.; Shipeng, W.; Li, L. Graphene oxide-supported zinc oxide nanoparticles for chloroprene rubber with improved crosslinking network and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2019, 124, 2–9. [Google Scholar] [CrossRef]
- Lingmin, K.; Yong, Z.; Guangsu, H.; Jinrong, W. Carbon nanodots as dual role of crosslinking and reinforcing chloroprene rubber. Compos. Commun. 2020, 22, 1–8. [Google Scholar]
- Shaojian, H.; Tengfei, H.; Jiaqi, W.; Xiaohui, W.; Yang, X.; Liqun, Z.; Jun, L. A novel method to prepare acrylonitrile-butadiene rubber/clay nanocomposites by compounding with clay gel. Compos. Part. B Eng. 2019, 167, 356–361. [Google Scholar]
- Uddin, F. Clays, nanoclays, and montmorillonite minerals. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2008, 39, 2804–2814. [Google Scholar] [CrossRef]
- Das, A.; Mahaling, R.N.; Stöckelhuber, K.W.; Heinrich, G. Reinforcement and migration of nanoclay in polychloroprene/ethylene-propylene-diene-monomer rubber blends. Compos. Sci. Technol. 2011, 71, 276–281. [Google Scholar] [CrossRef]
- Urrego Yepes, W.; Velásquez Restrepo, S.M.; Giraldo Vásquez, D.H.; Posada Correo, J.C. Revisión- Efecto del sistema de vulcanizacion en la red entrecruzada y en la reacción química de vulcanización del caucho natural. Rev. EIA 2017, 14, 99–115. [Google Scholar] [CrossRef] [Green Version]
- Annadurai, P.; Mukundan, T.; Joseph, R. Influence of carbon black in polychloroprene organoclay nanocomposite with improved mechanical, electrical and morphology characteristics. Plast. Rubber Compos. 2013, 42, 379–384. [Google Scholar] [CrossRef]
- Mathew, G.; Rhee, J.M.; Lee, Y.S.; Park, D.H.; Nah, C. Cure kinetics of ethylene acrylate rubber/clay nanocomposites. J. Ind. Eng. Chem. 2008, 14, 60–65. [Google Scholar] [CrossRef]
- Romli, A.Z.; Mamauod, S.N.L. Physical and Mechanical Properties of ENR Compatibilized NR/NBR Blends Reinforced Nanoclay and Nanosilica. Macromol. Symp. 2017, 371, 27–34. [Google Scholar] [CrossRef]
- Praveen, S.; Chattopadhyay, P.K.; Albert, P.; Dalvi, V.G.; Chakraborty, B.C.; Chattopadhyay, S. Synergistic effect of carbon black and nanoclay fillers in styrene butadiene rubber matrix: Development of dual structure. Compos. Part A Appl. Sci. Manuf. 2009, 40, 309–316. [Google Scholar] [CrossRef]
- Etika, K.C.; Liu, L.; Hess, L.A.; Grunlan, J.C. The influence of synergistic stabilization of carbon black and clay on the electrical and mechanical properties of epoxy composites. Carbon N. Y. 2009, 47, 3128–3136. [Google Scholar] [CrossRef]
- Gent, A.; Pulford, C.T. Mechanism of Rubber Abrasion. J. Appl. Polym. Sci. 1983, 28, 943–960. [Google Scholar] [CrossRef]
- Raji Vijay, V.; Anitha, A.M.; Ravindranatha Menon, A.R. Studies on blends of natural rubber and butadiene rubber containing silica-Organomodified kaolin hybrid filler systems. Polymer 2016, 89, 135–142. [Google Scholar] [CrossRef]
- Sreelekshmi, R.V.; Brahmakumar, M.; Sudha, J.D.; Ravindranatha Menon, A.R. Studies on natural rubber containing kaolin modified with hexamethylenediamine derivative of phosphorylated cashew nut shell liquid prepolymer. Appl. Clay. Sci. 2017, 141, 171–179. [Google Scholar] [CrossRef]
- Tabsan, N.; Wirasate, S.; Suchiva, K. Abrasion behavior of layered silicate reinforced natural rubber. Wear 2010, 269, 394–404. [Google Scholar] [CrossRef]
- Lin, Z.; Joseph, T.G.; Curley, M. Specific energy and the modified rubber wheel abrasion test. Wear 2017, 370–371, 9–16. [Google Scholar] [CrossRef]
- Mohomane, S.M.; Djokovic, V.; Thomas, S.; Luyt, A.S. Polychloroprene nanocomposites filled with different organically modified clays: Morphology, thermal degradation and stress relaxation behaviour. Polym. Test. 2011, 30, 585–593. [Google Scholar] [CrossRef]
- Das, A.; Costa, F.R.; Wagenknecht, U.; Heinrich, G. Nanocomposites based on chloroprene rubber: Effect of chemical nature and organic modification of nanoclay on the vulcanizate properties. Eur. Polym. J. 2008, 44, 3456–3465. [Google Scholar] [CrossRef]
- Boukerrou, A.; Duchet, J.; Fellahi, S.; Djidjelli, H.; Kaci, M.; Sautereau, H. Synthesis and characterization of rubbery epoxy/organoclay hectorite nanocomposites. Express Polym. Lett. 2007, 1, 824–830. [Google Scholar] [CrossRef]
- Belluci, F.; Camino, G.; Frache, A.; Sarra, A. Catalytic charring-volatizatin competition in organoclay nanocomposites. Polym. Degrad. Stab. 2007, 92, 425–436. [Google Scholar] [CrossRef]
- Xie, W.; Gao, Z.; Liu, K.; Pan, W.P.; Vaia, R.; Hunter, D.; Singh, A. Thermal characterization of organically modified montmorillonite. Thermochim. Acta 2001, 367, 339–350. [Google Scholar] [CrossRef]
- Lu, F.J.; Hsu, S.L. Vibrational Spectroscopic Analysis of the Structure of Natural Rubber. Rubber Chem. Technol. 1987, 60, 647–658. [Google Scholar] [CrossRef]
- Nallasamy, P.; Mohan, S. Vibrational Spectra of Cis-1, 4-Polyisoprene. Arab. J. Sci. Eng. 2004, 29, 17–26. [Google Scholar]
- Rolere, S.; Liengprayoon, S.; Vaysse, L.; Sainte-Beuve, J.; Bonfils, F. Investigating natural rubber composition with Fourier Transform Infrared (FT-IR) spectroscopy: A rapid and non-destructive method to determine both protein and lipid contents simultaneously. Polym. Test. 2015, 43, 83–93. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarma, A.D.; Le, H.H.; Das, A.; Wießner, S.; Stöckelhuber, K.W.; Bhowmick, A.K.; Heinrich, G. Determination of phase specific localization of carbon black in ternary rubber blends: A macroscopic approach by fourier transform infrared spectroscopy (FTIR). Polymer 2018, 150, 64–71. [Google Scholar] [CrossRef]
- Dimitry, O.I.H.; Abdeen, Z.; Ismail, E.A.; Saad, A.L.G. Studies of particle dispersion in elastomeric polyurethane/organically modified montmorillonite nanocomposites. Int. J. Green Nanotechnol. 2011, 3, 197–212. [Google Scholar] [CrossRef]
- Pack, S.; Kashiwagi, T.; Cao, C.; Korach, C.S.; Lewin, M.; Rafailovich, M.H. Role of surface interactions in the synergizing polymer/clay flame retardant properties. Macromolecules 2010, 43, 5338–5351. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Sinegovskaya, L.M.; Gusarova, N.K. Journal of Sulfur Chemistry Vibrations of the S–S bond in elemental sulfur and organic polysulfides: A structural guide. J. Sulfur. Chem. 2009, 30, 518–554. [Google Scholar] [CrossRef]
- Maji, P.K.; Guchhait, P.K.; Bhowmick, A.K. Effect of the microstructure of a hyperbranched polymer and nanoclay loading on the morphology and properties of novel polyurethane nanocomposites. ACS Appl. Mater. Interfaces 2009, 1, 289–300. [Google Scholar] [CrossRef]





| Filler | Intergallery Spacing d(001) (Å) | Organic Modifier | Particle Size (nm) |
|---|---|---|---|
| Organoclay Cloisite 10 A (C10A) | 19.0 | Dimethyl, benzyl, hydrogenated tallow, quaternary ammonium chloride. | - |
| Organoclay Cloisite 15 (C15) | 36.3 | Dimethyl, dehydrogenated tallow, quaternary ammonium chloride. | - |
| Carbon black N330 (CB) | - | - | 29 |
| Composite Code | Chloroprene Rubber (CR) | Natural Rubber (NR) | Butadiene Rubber (BR) | Cloisite® 10 A (C10A) | Cloisite® 15 (C15) | Carbon Black (CB) | Zinc Oxide (ZnO) | S | Others |
|---|---|---|---|---|---|---|---|---|---|
| A0 | 69.6 | 25.3 | 5.1 | 0.0 | 0.0 | 0.0 | 2.8 | 2.2 | 6.8 |
| A10-3 | 69.6 | 25.3 | 5.1 | 3.0 | 0.0 | 0.0 | 2.8 | 2.2 | 6.8 |
| A10-5 | 69.6 | 25.3 | 5.1 | 5.0 | 0.0 | 0.0 | 2.8 | 2.2 | 6.8 |
| A10-7 | 69.6 | 25.3 | 5.1 | 7.0 | 0.0 | 0.0 | 2.8 | 2.2 | 6.8 |
| A10-10 | 69.6 | 25.3 | 5.1 | 10.0 | 0.0 | 0.0 | 2.8 | 2.2 | 6.8 |
| A15-3 | 69.6 | 25.3 | 5.1 | 0.0 | 3.0 | 0.0 | 2.8 | 2.2 | 6.8 |
| A15-5 | 69.6 | 25.3 | 5.1 | 0.0 | 5.0 | 0.0 | 2.8 | 2.2 | 6.8 |
| A15-7 | 69.6 | 25.3 | 5.1 | 0.0 | 7.0 | 0.0 | 2.8 | 2.2 | 6.8 |
| A15-10 | 69.6 | 25.3 | 5.1 | 0.0 | 10.0 | 0.0 | 2.8 | 2.2 | 6.8 |
| B0 | 69.6 | 25.3 | 5.1 | 0.0 | 0.0 | 40.0 * | 2.8 | 2.2 | 6.8 |
| B10-3 | 69.6 | 25.3 | 5.1 | 3.0 | 0.0 | 40.0 * | 2.8 | 2.2 | 6.8 |
| B10-5 | 69.6 | 25.3 | 5.1 | 5.0 | 0.0 | 40.0 * | 2.8 | 2.2 | 6.8 |
| B10-7 | 69.6 | 25.3 | 5.1 | 7.0 | 0.0 | 40.0 * | 2.8 | 2.2 | 6.8 |
| B10-10 | 69.6 | 25.3 | 5.1 | 10.0 | 0.0 | 40.0 * | 2.8 | 2.2 | 6.8 |
| B15-3 | 69.6 | 25.3 | 5.1 | 0.0 | 3.0 | 40.0 * | 2.8 | 2.2 | 6.8 |
| B15-5 | 69.6 | 25.3 | 5.1 | 0.0 | 5.0 | 40.0 * | 2.8 | 2.2 | 6.8 |
| B15-7 | 69.6 | 25.3 | 5.1 | 0.0 | 7.0 | 40.0 * | 2.8 | 2.2 | 6.8 |
| B15-10 | 69.6 | 25.3 | 5.1 | 0.0 | 10.0 | 40.0 * | 2.8 | 2.2 | 6.8 |
| Composite Code | Tensile Strength (MPa) | Elongation at Break (%) | M 300 (MPa) | Tear Strength (N mm−1) |
|---|---|---|---|---|
| A0 | 5.36 ± 1.52 | 730.91 ± 95.97 | 1.35 ± 0.03 | 16.29 ± 1.56 |
| A10-3 | 6.85 ± 0.80 | 739.10 ± 41.61 | 1.74 ± 0.04 | 21.08 ± 0.80 |
| A10-5 | 11.85 ± 1.09 | 832.15 ± 29.95 | 2.41 ± 0.36 | 24.05 ± 1.20 |
| A10-7 | 13.65 ± 0.76 | 836.82 ± 11.38 | 3.05 ± 0.05 | 31.51 ± 2.29 |
| A10-10 | 10.14 ± 0.79 | 768.33 ± 23.77 | 2.88 ± 0.07 | 30.76 ± 1.91 |
| A15-3 | 9.95 ± 1.13 | 840.35 ± 37.73 | 1.88 ± 0.03 | 19.09 ± 0.87 |
| A15-5 | 13.42 ± 0.83 | 933.19 ± 45.89 | 2.24 ± 0.20 | 25.13 ± 1.45 |
| A15-7 | 12.40 ± 0.85 | 915.79 ± 13.82 | 2.31 ± 0.13 | 28.82 ± 2.84 |
| A15-10 | 10.01 ± 0.90 | 797.01 ± 41.74 | 2.70 ± 0.07 | 26.58 ± 2.32 |
| B0 | 9.95 ± 0.84 | 477.64 ± 15.41 | 6.71 ± 0.18 | 27.97 ± 0.66 |
| B10-3 | 8.55 ± 0.82 | 338.55 ± 22.87 | 7.49 ± 0.15 | 26.64 ± 2.49 |
| B10-5 | 7.44 ± 1.01 | 519.05 ± 6.81 | 6.54 ± 0.13 | 27.42 ± 2.68 |
| B10-7 | 10.73 ± 0.72 | 399.15 ± 16.38 | 7.74 ± 0.35 | 29.83 ± 0.46 |
| B10-10 | 8.83 ± 1.10 | 370.92 ± 31.69 | 6.86 ± 0.18 | 28.73 ± 2.82 |
| B15-3 | 12.77 ± 0.71 | 466.23 ± 25.73 | 7.19 ± 0.22 | 26.87 ± 2.53 |
| B15-5 | 7.02 ± 0.63 | 455.75 ± 17.58 | 6.35 ± 0.09 | 29.38 ± 2.14 |
| B15-7 | 12.43 ± 0.40 | 470.10 ± 18.88 | 6.97 ± 0.14 | 30.85 ± 2.23 |
| B15-10 | 7.31 ± 0.02 | 310.98 ± 1.47 | 7.11 ± 0.10 | 33.06 ± 2.56 |
| Composite Code | Hardness (Shore A) | Loss of Volume by Abrasion (mm3) | Reduction of Volume Loss (%) | Density (g/cm3) |
|---|---|---|---|---|
| A0 | 42 ± 0 | 449.97 ± 5.88 | - | 1.1356 ± 0.0011 |
| A10-3 | 47 ± 0 | 251.29 ± 13.09 | 44.15 | 1.1479 ± 0.0026 |
| A10-5 | 51 ± 0 | 243.42 ± 9.81 | 45.96 | 1.1497 ± 0.0022 |
| A10-7 | 56 ± 1 | 251.35 ± 7.13 | 44.14 | 1.1460 ± 0.0051 |
| A10-10 | 54 ± 1 | 269.68 ± 1.37 | 40.06 | 1.1607 ± 0.0042 |
| A15-3 | 50 ± 1 | 246.23 ± 5.05 | 45.28 | 1.1444 ± 0.0010 |
| A15-5 | 54 ± 1 | 241.73 ± 7.75 | 46.29 | 1.1479 ± 0.0022 |
| A15-7 | 62 ± 1 | 251.99 ± 15.73 | 43.99 | 1.1518 ± 0.0016 |
| A15-10 | 53 ± 0 | 399.11 ± 26.92 | 11.3 | 1.1587 ± 0.0004 |
| B0 | 59 ± 1 | 288.86 ± 6.69 | - | 1.2016 ± 0.0007 |
| B10-3 | 61 ± 1 | 242.07 ± 2.62 | 16.2 | 1.2101 ± 0.0016 |
| B10-5 | 64 ± 1 | 243.56 ± 11.15 | 15.68 | 1.2121 ± 0.0064 |
| B10-7 | 61 ± 1 | 240.51 ± 8.38 | 16.74 | 1.2164 ± 0.0015 |
| B10-10 | 63 ± 0 | 266.96 ± 3.72 | 7.51 | 1.2197 ± 0.0010 |
| B15-3 | 63 ± 1 | 227.12 ± 2.30 | 21.37 | 1.2040 ± 0.0013 |
| B15-5 | 66 ± 1 | 247.14 ± 12.32 | 14.44 | 1.2088 ± 0.0030 |
| B15-7 | 63 ± 0 | 216.48 ± 3.35 | 25.06 | 1.2186 ± 0.0004 |
| B15-10 | 64 ± 0 | 249.51 ± 3.86 | 13.62 | 1.2124 ± 0.0014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castaño-Rivera, P.; Calle-Holguín, I.; Castaño, J.; Cabrera-Barjas, G.; Galvez-Garrido, K.; Troncoso-Ortega, E. Enhancement of Chloroprene/Natural/Butadiene Rubber Nanocomposite Properties Using Organoclays and Their Combination with Carbon Black as Fillers. Polymers 2021, 13, 1085. https://doi.org/10.3390/polym13071085
Castaño-Rivera P, Calle-Holguín I, Castaño J, Cabrera-Barjas G, Galvez-Garrido K, Troncoso-Ortega E. Enhancement of Chloroprene/Natural/Butadiene Rubber Nanocomposite Properties Using Organoclays and Their Combination with Carbon Black as Fillers. Polymers. 2021; 13(7):1085. https://doi.org/10.3390/polym13071085
Chicago/Turabian StyleCastaño-Rivera, Patricia, Isabel Calle-Holguín, Johanna Castaño, Gustavo Cabrera-Barjas, Karen Galvez-Garrido, and Eduardo Troncoso-Ortega. 2021. "Enhancement of Chloroprene/Natural/Butadiene Rubber Nanocomposite Properties Using Organoclays and Their Combination with Carbon Black as Fillers" Polymers 13, no. 7: 1085. https://doi.org/10.3390/polym13071085