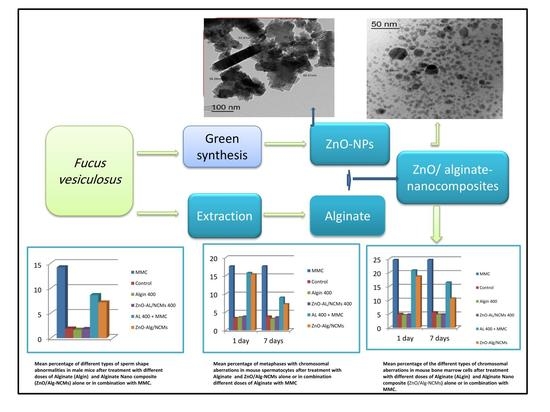
Assessment of the Antigenotoxic Effects of Alginate and ZnO/Alginate–Nanocomposites Extracted from Brown Alga Fucus vesiculosus in Mice
Abstract
:1. Introduction
2. Material and Methods
2.1. Alga
2.2. Alginate Extraction
2.3. Alginate Yield
2.4. Alginates Characterisation
2.5. Green Synthesis of Zinc Oxide Nanoparticles
2.6. Synthesis of ZnO/Alginates Nanocomposites (ZnO/Alg-NCMs)
2.7. Characterizations of Zinc Oxide Nanoparticles and ZnO/Alg-NCMs
2.8. Chromosome Abnormalities in Bone Marrow Cells
2.9. Chromosome Abnormalities in Spermatocytes
2.10. Sperm Morphology Assay
2.11. Statistical Analysis
3. Results and Discussion
3.1. Alginate Yields
3.2. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
3.3. Electrospray Mass Spectrometer
3.4. Proton Nuclear Magnetic Resonance (H1 NMR) Analysis
3.5. SEM and TEM Image
3.6. Zeta Potential
3.7. Energy Dispersive X-ray Measurements
3.8. Chromosomal Aberrations in Bone Marrow Cells
3.9. Chromosome Abnormalities in Spermatocytes
3.10. Sperm Shape Abnormalities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, Y.; Shang, Q.; Li, W.; Guo, W.; Stojadinovic, A.; Mannion, C.; Man, Y.; Chen, T. Antibiotics for cancer treatment: A double-edged sword. J. Cancer 2020, 11, 5135–5149. [Google Scholar] [CrossRef]
- Tomasz, M. Mitomycin C: Small, fast and deadly (but very selective). Chem. Biol. 1995, 2, 575–579. [Google Scholar] [CrossRef] [Green Version]
- Saif, M.W.; Kaley, K.; Brennan, M.; Garcon, M.C.; Rodriguez, G. Mitomycin-C and Capecitabine (MIXE) as Salvage Treatment in Patients with Refractory Metastatic Colorectal Cancer:A Retrospective Study. Anticancer Res. 2013, 33, 2743–2746. [Google Scholar]
- Sinitsky, M.Y.; Kutikhin, A.G.; Tsepokina, A.V.; Shishkova, D.K.; Asanov, M.A.; Yuzhalin, A.E.; Minina, V.I.; Ponasenko, A.V. Mitomycin C induced genotoxic stress in endothelial cells is associated with differential expression of proinflammatory cytokines. Mutat. Res.-Genet. Toxicol. Environ. Mutagenes. 2020, 858–860, 503252. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.K. Functional foods, herbs and nutraceuticals: Towards biochemical mechanisms of healthy aging. Biogerontology 2004, 5, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Ansari, R.; Sadati, S.M.; Mozafari, N.; Ashrafi, H.; Azadi, A. Carbohydrate polymer-based nanoparticle application in drug delivery for CNS-related disorders. Eur. Polym. J. 2020, 128, 109607. [Google Scholar] [CrossRef]
- Dong, Q.Y.; Chen, M.Y.; Xin, Y.; Qin, X.Y.; Cheng, Z.; Shi, L.E.; Tang, Z.X. Alginate-based and protein-based materials for probiotics encapsulation: A review. Int. J. Food Sci. Technol. 2013, 48, 1339–1351. [Google Scholar] [CrossRef]
- Comaposada, J.; Gou, P.; Marcos, B.; Arnau, J. Physical properties of sodium alginate solutions and edible wet calcium alginate coatings. LWT-Food Sci. Technol. 2015, 64, 212–219. [Google Scholar] [CrossRef]
- Paulsen, B. Plant polysaccharides with immunostimulatory activities. Curr. Org. Chem. 2001, 5, 939–950. [Google Scholar] [CrossRef]
- Truus, K.; Vaher, M.; Taure, I. Algal biomass from Fucus vesiculosus (Phaeophyta): Investigation of the mineral and alginate components. Proc. Est. Acad. Sci. Chem. 2001, 50, 95–103. [Google Scholar]
- Tusi, S.K.; Khalaj, L.; Ashabi, G.; Kiaei, M.; Khodagholi, F. Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial mediated apoptotic cell death and oxidative stress. Biomaterials 2011, 32, 5438–5458. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Xu, F.Q.; Li, Y.H.; Li, J.; Liu, X.; Wang, X.F.; Hu, L.G.; An, Y. Alginate oligosaccharide alleviates myocardial reperfusion injury by inhibiting nitrative and oxidative stress and endoplasmic reticulum stress mediated apoptosis. Drug Des. Dev. Ther. 2017, 11, 2387–2397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Ma, J.L.; Shi, H.; Zhu, J.B.; Wu, J.; Ding, Z.W.; An, Y.; Zou, Y.; Ge, J.B. Alginate oligosaccharide prevents acute doxorubicin cardiotoxicity by suppressing oxidative stress and endoplasmic reticulum-mediated apoptosis. Mar. Drugs 2016, 14, 231. [Google Scholar] [CrossRef] [Green Version]
- Keweloh, H.; Heipieper, H.J.; Rehm, H.J. Protection of bacteria against toxicity of phenol by immobilization in calcium alginate. Appl. Microbiol. Biotechnol. 1989, 31, 383–389. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Zhang, L.; Wang, Y.; Wang, S.; Zhang, Y.; Guo, H.; Ji, D.; Wang, Y. Alginate oligosaccharide DP5 exhibits antitumor effects in osteosarcoma patients following surgery. Front. Pharmacol. 2017, 8, 623. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, X.; Hwang, H.; Liu, S.; Guan, H. Antitumour activities of alginate-derived oligosaccharides and their sulphated substitution derivatives. Eur. J. Phycol. 2004, 39, 67–71. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, L.; Yu, X.; Wang, S.; Xu, C.; Yin, H.; Wang, S. Alginate oligosaccharide attenuates α2,6-sialylation modification to inhibit prostate cancer cell growth via the Hippo/YAP pathway. Cell Death Dis. 2019, 10, 374. [Google Scholar] [CrossRef] [Green Version]
- Manzelat, S.F.; Mufarrah, A.M.; Hasan, B.A.; Hussain, N.A. Macro algae of the Red Sea from Jizan, Saudi Arabia. Phykos 2018, 48, 88–108. [Google Scholar]
- Al-Asmar, A.; Giosafatto, C.V.L.; Sabbah, M.; Sanchez, A.; Santana, R.V.; Mariniello, L. Effect of Mesoporous Silica Nanoparticles on the Physicochemical Properties of Pectin Packaging Material for Strawberry Wrapping. Nanomaterials 2020, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Hessien, M.; Da’na, E.; Taha, A. Phytoextract Assisted Hydrothermal Synthesis of ZnO–NiO Nanocomposites Using Neem Leaves Extract. Ceram. Int. 2021, 47, 811–816. [Google Scholar] [CrossRef]
- Karnan, T.; Selvakumar, S.A.S. Biosynthesis of ZnO Nanoparticles Using Rambutan (Nephelium Lappaceum L.) Peel Extract and Their Photocatalytic Activity on Methyl Orange Dye. J. Mol. Struct. 2016, 1125, 358–365. [Google Scholar] [CrossRef]
- Ahmed, S.; Chaudhry, S.A.; Ikram, S.A. Review on Biogenic Synthesis of ZnO Nanoparticles Using Plant Extracts and Microbes: A Prospect towards Green Chemistry. J. Photochem. Photobiol. B 2017, 166, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Sabela, M.I.; Kanchi, S.; Mdluli, P.S.; Singh, G.; Stenström, T.A.; Bisetty, K. Biosynthesis of ZnO Nanoparticles Using Jacaranda mimosifolia Flowers Extract: Synergistic Antibacterial Activity and Molecular Simulated Facet Specific Adsorption Studies. J. Photochem. Photobiol. B 2016, 162, 199–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakil, S.N.A.; Kamal, H.; Abdullah, H.Z.; Idris, M.I. Sodium alginate-zinc oxide nanocomposite film for antibacterial wound healing applications. Biointerface Res. Appl. Chem. 2020, 10, 6289–6296. [Google Scholar]
- Choi, J.; Park, Y.G.; Yun, M.S.; Seol, J.W. Effect of herbal mixture composed of Alchemilla vulgaris and Mimosa on wound healing process. Biomed. Pharmacother. 2018, 106, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, M.G.; Karimian, R.; Rakhshaei, R.; Pakdel, F.; Eslami, H.; Fakhrzadeh, V.; Rahimi, M.; Salehi, R.; Kafil, H.S. Chitin/silk fibroin/TiO2 bio-nanocomposite as a biocompatible wound dressing bandage with strong antimicrobial activity. Int. J. Biol. Macromol. 2018, 116, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Heinze, T.; Hipler, U.C. Comparative in vitro study on cytotoxicity, antimicrobial activity, and binding capacity for pathophysiological factors in chronic wounds of alginate and silver-containing alginate. Wound Repair Regen. 2009, 17, 511–521. [Google Scholar] [CrossRef]
- Agren, M.S. Zinc in wound repair. Arch. Dermatol. 1999, 135, 1273–1274. [Google Scholar] [CrossRef]
- Taylor, F.G. Taxonomy and relatonships of red tide fagellates. In Toxic Dinofagellates; Anderson, D.M., White, A.W., Baden, D.G., Eds.; Elsevier: New York, NY, USA, 1985. [Google Scholar]
- Hambali, E.; Sakti, S.C.W.; Fahmi, M.Z.; Wahyudianto, F.E.; Yessi, P.; Yani, M.; Pratama, B.S. Effect of Extraction Time and Na2CO3 Concentration on The Characteristics of Alginate Extracted from Sargassum sp. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 209. [Google Scholar]
- Hamouda, R.A.; Yousuf, W.E.; Mohammed, A.A.; Darwish, D.B.; Abdeen, E.E. Comparative study between zinc oxide nanoparticles synthesized by chemical and biological methods in view of characteristics, antibacterial activity and loading on antibiotics in vitro. Dig. J. Nanomater. Biostruct. 2020, 15, 1–10. [Google Scholar]
- Hamouda, R.A.; Yousuf, W.E.; Mohammed, A.A.; Mohammed, R.S.; Darwish, D.B.; Abdeen, E.E. Comparative study between zinc oxide nanoparticles synthesis by biogenic and wet chemical methods in vivo and in vitro against Staphylococcus aureus. Microb. Pathog. 2020, 147, 104384. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.D.; Patlolla, A.K.; Tchounwou, P.B. Cytogenetic evaluation of malathion induced toxicity in Sprague-Dawley rats. Mutat. Res. 2011, 725, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Yosida, T.H.; Amano, K. Autosomal polymorphism in laboratory bred and wild Norway rats, Rattus norvegicus. Misima Chromosom. 1965, 16, 658–667. [Google Scholar] [CrossRef]
- Evans, E.P.; Breckon, G.; Ford, C.E. An air-drying method for meiotic preparations for mammalian testes. Cytogenetics 1964, 3, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Rasgele, P.G. Abnormal sperm morphology in mouse germ cells after short-term exposures to acetamiprid, propineb, and their mixture. Arch. Ind. Hyg. Toxicol. 2014, 65, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Wyrobek, A.J.; Bruce, W.R. The induction of sperm-shape abnormalities in mice and humans. In Chemical Mutagens: Principles and Methods for Their Detection; Hallaender, A., De Serres, F.J., Eds.; Plenum: New York, NY, USA, 1978; Volume 5, pp. 257–285. [Google Scholar]
- Rinaudo, M. Biomaterials based on a natural polysaccharide: Alginate. TIP 2014, 17, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- McHugh, D.J. Seaweeds used as a source of alginate, In: A Guide to the Seaweed Industry. FAO Fish. Tech. Pap. 2003, 441, 27–49. [Google Scholar]
- Łabowska, M.B.; Michalak, I.; Detyna, J. Methods of extraction, physicochemical properties of alginates and their applications in biomedical field–A review. Open Chem. 2019, 17, 738–762. [Google Scholar] [CrossRef] [Green Version]
- Mossoba, M.M.; Al-Khaldi, S.F.; Kirkwood, J.; Fry, F.S.; Sedman, J.; Ismail, A.A. Printing microarrays of bacteria for identification by infrared microspectroscopy. Vib. Spectrosc. 2005, 38, 229–235. [Google Scholar] [CrossRef]
- Rosu, D.; Rosu, L.; Cascaval, C.N. IR-change and yellowing of polyurethane as a result of UV irradiation. Polym. Degrad. Stab. 2009, 94, 591–596. [Google Scholar] [CrossRef]
- Munajad, A.; Subroto, C.; Suwarno, A. Fourier transform infrared spectroscopy (FTIR) analysis of transformer insulation paper in natural ester. In Proceedings of the 2017 International Conference on High Voltage Engineering and Power Systems (ICHVEPS), Denpasar, Indonesia, 2–5 October 2017; pp. 446–450. [Google Scholar] [CrossRef]
- Chiriboga, P.; Xie, H.; Yee, V.; Vigorita, D.; Zarou, D.; Zakim, D. Infrared spectroscopy of human tissue. I. Differentiation and maturation of epithelial cells in the human cervix. Biospectroscopy 1998, 4, 47–53. [Google Scholar] [CrossRef]
- Wong, P.T.T.; Papavassiliou, E.D.; Rigas, B. Phosphodiesterstretching bands in the infrared spectra of human tissues and cultured cells. Appl. Spectrosc. 1998, 45, 1563–1567. [Google Scholar] [CrossRef]
- Choo, L.P.; Mansfield, J.R.; Pizzi, N.; Somorjai, R.L.; Jackson, M.; Halliday, W.C.; Mantsch, H.H. Infrared spectra of human central nervous system tissue: Diagnosis of alzheimer’s disease by multivariate analyses. Biospectros 1995, 1, 141–148. [Google Scholar] [CrossRef]
- Sakakibara, A.; Sano, Y. Chemistry of lignin. Wood Cellul. Chem. 2000, 2, 109–173. [Google Scholar]
- Li, Y.M.; Sun, S.Q.; Zhou, Q.; Qin, Z.; Tao, J.X.; Wang, J.; Fang, X. Identification of American ginseng from different regions using FT-IR and two-dimensional correlation IR spectroscopy. Vib. Spectrosc. 2004, 36, 227–232. [Google Scholar] [CrossRef]
- Margoshes, M.; Fassel, V.A. The infrared spectra of aromatic compounds: I. The out-of-plane CH bending vibrations in the region 625–900 cm−1. Spectrochim. Acta. 1955, 7, 14–24. [Google Scholar] [CrossRef]
- Berthomieu, C.; Nabedryk, E.; Mäntele, W.; Breton, J. Characterization by FTIR spectroscopy of the photoreduction of the primary quinone acceptor QA in photosystem II. FEBS Lett. 2001, 269, 363–367. [Google Scholar] [CrossRef] [Green Version]
- Ren, F.; Ding, Y.; Leng, Y. Infrared spectroscopic characterization of carbonated apatite: A combined experimental and computational study. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2014, 102, 496–505. [Google Scholar] [CrossRef]
- Colagar, A.H.; Chaichi, M.J.; Khadjvand, T. Fourier transform infrared microspectroscopy as a diagnostic tool for distinguishing between normal and malignant human gastric tissue. J. Biosci. 2011, 36, 669–677. [Google Scholar] [CrossRef]
- Li, C.; Xin, Q. Direct observation of chemisorbed methane on cerium (IV) oxide by FTIR spectroscopy. J. Chem. Soc. Chem. Commun. 1992, 10, 782–783. [Google Scholar] [CrossRef]
- Gáplovská, K.; Šimonovičová, A.; Halko, R.; Okenicová, L.; Žemberyová, M.; Čerňanský, S.; Mackuľak, T. Study of the binding sites in the biomass of Aspergillus niger wild-type strains by FTIR spectroscopy. Chem. Papers. 2018, 72, 2283–2288. [Google Scholar] [CrossRef]
- Belattmania, Z.; Kaidi, S.; El Atouani, S.; Katif, C.; Bentiss, F.; Jama, C.; Vasconcelos, V. Isolation and FTIR-ATR and 1H NMR characterization of alginates from the main alginophyte species of the Atlantic Coast of Morocco. Molecules 2020, 25, 4335. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Anastyuk, S.D.; Surits, V.V.; Shevchenko, N.M.; Thinh, P.D.; Zadorozhny, P.A.; Ermakova, S.P. Comparison of structure and in vitro anticancer activity of native and modified fucoidans from Sargassum feldmannii and S. duplicatum. Int. J. Biol. Macromol. 2019, 124, 220–228. [Google Scholar] [CrossRef]
- Helmiyati, H.; Wahyuningrum, K.D. Synthesis and photocatalytic activity of nanocomposite based on sodium alginate from brown algae with ZnO impregnation. In AIP Conference Proceedings; AIP Publishing LLC.: Melville, NY, USA, 2018; Volume 2023. [Google Scholar]
- Trandafilović, L.V.; Božanić, D.K.; Dimitrijević-Branković, S.; Luyt, A.S.; Djoković, V. Fabrication and antibacterial properties of ZnO–alginate nanocomposites. Carbohydr. Polym. 2012, 88, 263–269. [Google Scholar] [CrossRef]
- Emamifar, A.; Bavaisi, S. Nanocomposite coating based on sodium alginate and nano-ZnO for extending the storage life of fresh strawberries (Fragaria× ananassa Duch.). J. Food Meas. Charact. 2020, 2012, 1–13. [Google Scholar] [CrossRef]
- Pomastowski, P.; Król-Górniak, A.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanocomposites-Extracellular synthesis, physicochemical characterization and antibacterial potential. Materials 2020, 13, 4347. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.J.; Mehdi, M.S. Study of morphology and zeta potential analyzer for the silver nanoparticles. Int. J. Sci. Eng. Res. 2014, 5, 381–385. [Google Scholar]
- Farhadi, S.; Ajerloo, B.; Mohammadi, A. Green biosynthesis of spherical silver nanoparticles by using date palm (phoenix dactylifera) fruit extract and study of their antibacterial and catalytic activities. Acta Chim. Slov. 2017, 64, 129–143. [Google Scholar] [CrossRef]
- Keshavarz, M.; Alizadeh, P. On the role of alginate coating on the mechanical and biological properties of 58S bioactive glass scaffolds. Int. J. Biol. Macromol. 2021, 167, 947–961. [Google Scholar] [CrossRef]
- Tucker, J.D.; Preston, B.J. Chromosome aberrations, micronuclei, aneuploidy, sister chromatid exchanges and cancer risk assessment. Mut. Res. 1996, 365, 147–159. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.A.; El-Nekeety, A.A.; Salman, A.S.; Abdel-Aziem, S.H.; Mehaya, F.M.; Hassan, N.S. Protective capabilities of silymarin and inulin nanoparticles against hepatic oxidative stress, genotoxicity and cytotoxicity of Deoxynivalenol in rats. Toxicon 2018, 142, 1–13. [Google Scholar] [CrossRef]
- Khalil, W.K.B.; Ahmed, E.S.; Kassem, S.M.; Shoman, T.M.T.; Hassanane, M.M.; Eshak, M.G. Antioxidant capacity of Nitraria retusa leaf extracts against mitomycin C-induced genetic toxicity in male mice. J. Basic Appl. Zool. 2019, 80, 26. [Google Scholar] [CrossRef]
- Morimoto, K.; Sato-Mizuno, M.; Koizumi, A. Sister-chromatid exchanges and cell cycle-kinetics in human lymphocytes cultures exposed to alkaylating mutagens: Apparent deformity in dose-response relationship. Mut. Res. 1985, 152, 187–196. [Google Scholar] [CrossRef]
- Bean, C.L.; Armstrong, M.J.; Galloway, S.M. Effects of sampling time on chromosome aberration yield for seven chemicals in Chinese hamster ovary cells. Mut. Res. 1992, 265, 31–44. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, S.J.; Ciccone, S.L.M.; Kim, C.R.; Lee, S.H. An in vivo analysis of MMC-induced DNA damage and its repair. Carcinogenesis 2006, 27, 446–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paz, M.M. Reductive activation of mitomycin C by thiols: Kinetics, mechanism, and biological implications. Chem. Res. Toxicol. 2009, 22, 1663–1668. [Google Scholar] [CrossRef]
- Majumdar, S.K.; Buchanan, B.; Feinsein, S. Mutagenicity studies with 5-thio-D-glucose. J. Hered. 1979, 70, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.; Linden, G. Observations sur les propriétés mutagéniques des cyclamates ches les mammiferes. C. R. Soc. Biol. 1972, 166, 468–470. [Google Scholar]
- Liang, J.C.; Pacchierotti, F. Cytogenetic investigation of chemically-induced aneuploidy in mouse spermatocytes. Mut. Res. 1988, 201, 325–335. [Google Scholar] [CrossRef]
- Kumari, S.; Nayak, G.; Lukose, S.T.; Kalthur, S.G.; Bhatb, N.; Hegde, A.R.; Mutalik, S.; Kalthur, G.; Adiga, S.K. Indian propolis ameliorates the mitomycin C-induced testicular toxicity by reducing DNA damage and elevating the antioxidant activity. Biomed. Pharm. 2017, 95, 252–263. [Google Scholar] [CrossRef]
- Brusick, D. Principles of Genetic Toxicology; Plenum Press: New York, NY, USA, 1980. [Google Scholar]
- Topham, J.C. Chemically induced changes in sperm in animals and humans. Chem. Mutagen. 1983, 8, 201–234. [Google Scholar]
- Nystrom, C.; Bisrat, M. Physiochemical aspects of drug release VIII. The relation between particle size and surface specific dissolution rate in agitated suspensions. Int. J. Pharm. 1988, 47, 223–231. [Google Scholar]
- Anderberg, E.K.; Nystrom, C.; Bisrat, M. Physiochemical aspects of drug release VII. The effect of surfactant concentration and drug particle size on solubility and dissolution rate of felodipine, a sparingly soluble drug. Int. J. Pharm. 1988, 47, 67–77. [Google Scholar] [CrossRef]
- Mcneil, S.E. Nanotechnology for the biologist. J. Leukoc. Biol. 2005, 78, 585–592. [Google Scholar] [CrossRef]
- Kesisoglou, F.; Panmai, S.; Wu, Y. Nanosizing-oral formulation development and biopharmaceutical evaluation. Adv. Drug Deliv. Rev. 2007, 59, 631–644. [Google Scholar] [CrossRef] [PubMed]










| Alginic Acids Wavenumber [cm−1] | Alginates Wavenumber [cm−1] | Assignment | Ref. No. |
|---|---|---|---|
| 3448 | 3449 | OH bonds | [42] |
| 2923 | 2922 | Asymmetric stretching vibration of CH2 of acyl chains (lipids) | [32] |
| 2855 | 2854 | CH2 symmetric stretching | [5] |
| 1653 | C D O, C D N, NH of adenine, thymine, guanine, cytosine | [51] | |
| 1620 | 1625 | Amide II | [28] |
| 1519 | Amide II | ||
| 1465 | CH2 scissoring mode of the acyl chain of lipid | [52] | |
| 1418 | 1402 | d C–H, d C–O-H | [53] |
| 1308 | 1273 | CHaα– rocking | [54] |
| 1126 | 1115 | Symmetric stretching P–O–C | [47] |
| 1048 | 1048 | C=O groups | [55] |
| 892–819 | 870 | Vibrations of aromatic ring | |
| 781–725 | |||
| 675–622 | 672 | 800–600 C–Cl | [31,32] |
| - | 588 | 750–500 C–I | [31,32] |
| 433 | 528 | 750–500 C–I | [30,31] |
| Treatment and Doses (mg/kg b.wt.) | Treatment Day(s) | No. of Metaphases with | Chromosomal Aberrations | Inhibition % | |||||
|---|---|---|---|---|---|---|---|---|---|
| Gap | Frag. and/or Break | Gap+(Frag. or Break) | Deletion | Rt. | Excluding Gaps Mean ± S.E. | Including Gaps | |||
| MMC | 1 | 24 | 65 | 26 | 4 | 4 | 19.8 ± 0.26 a | 24.6 ± 0.4 a | |
| Control | 10 | 15 | - | - | 3.0 ± 0.2 | 5.0 ± 0.27 | |||
| Algin 400 | 7 | 14 | 2 | - | - | 3.2 ± 0.2 | 4.6 ± 0.21 | ||
| ZnO-ALg/NCMs 400 | 10 | 13 | 1 | - | - | 2.8 ± 0.21 | 4.8 ± 0.23 | ||
| AL 400 + MMC | 18 | 56 | 24 | 4 | 2 | 17.2 ± 0.7 a | 20.8 ±0.27 a | 15.44 | |
| ZnO-Alg/NCMs + MMC | 14 | 52 | 26 | 1 | - | 15.8 ± 0.45 a | 18.6 ±0.3 a | 24.39 | |
| MMC | 1 | 24 | 65 | 26 | 4 | 4 | 19.8 ± 0.26 a | 24.6 ± 0.4 a | |
| Control | 7 | 12 | 15 | 1 | - | - | 3.2 ± 0.37 | 5.6 ±0.3 | |
| Algin 400 | 11 | 13 | - | - | - | 2.6 ± 0.3 | 4.8 ± 0.23 | ||
| ZnO-Alg/NCMs 400 | 9 | 14 | 1 | - | - | 3.0 ± 0.3 | 4.8 ± 0.3 | ||
| AL 400 + MMC | 21 | 48 | 13 | - | - | 12.2 ± 0.3 ab | 16.4 ± 0.3 ab | 33.33 | |
| ZnO-Alg/NCMs + MMC | 15 | 24 | 14 | - | - | 7.6 ± 0.2 ab | 10.6 ± 0.34 ab | 56.9 | |
| Treatment and Doses (mg/kg b.wt.) | Treatment Day(s) | No. of Different Types of Chromosomal Aberrations | Total Aberrations | Inhibition % | |||
|---|---|---|---|---|---|---|---|
| XY Univalent | Autosomal Univalent | XY+ Autosomal Univalent | No. | Mean % ± S.E. | |||
| MMC | 1 | 46 | 27 | 15 | 88 | 17.6 ± 0.44 a | |
| Control | 13 | 4 | - | 17 | 3.4 ± 0.2 | ||
| AL 400 | 14 | 4 | - | 18 | 3.6 ± 0.24 | ||
| ZnO-Alg/NCMs 400 | 15 | 3 | 1 | 19 | 3.8 ± 0.2 | ||
| Alginate 400 + MMC | 42 | 27 | 10 | 79 | 15.8 ± 0.4 a | 10.2 | |
| ZnO-Alg/NCMs + MMC | 43 | 25 | 9 | 77 | 15.4 ± 0.36 a | 12.5 | |
| MMC | 1 | 46 | 27 | 15 | 88 | 17.6 ± 0.44 a | |
| Control | 7 | 13 | 6 | - | 19 | 3.8 ± 0.24 | |
| Alginate 400 | 12 | 4 | - | 16 | 3.2 ± 0.2 | ||
| ZnO-Alg/NCMs 400 | 13 | 5 | - | 18 | 3.6 ± 0.22 | ||
| Alginate 400 + MMC | 28 | 10 | 7 | 45 | 9.0 ± 0.5 ab | 48.8 | |
| ZnO-Alg/NCMs + MMC | 22 | 9 | 5 | 36 | 7.2 ± 0.33 ab | 59.09 | |
| Treatment and Doses (mg/kg b.wt.) | Examined Sperm No. | No. of Sperms with | Abnormal Sperm No. | Abnormal Sperms Mean % ± S.E. | Inhibition % | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Head Abnormalities | Tail Abnormalities | |||||||||
| Amorphous | Triangle | Without Hook | Small | Big | Coiled | |||||
| MMC | 5000 | 171 | 163 | 135 | 6 | 5 | 230 | 710 | 14.2 ± 0.21 a | |
| Control | 5000 | 34 | 27 | 19 | - | - | 15 | 95 | 1.9 ± 0.25 | |
| Alginate 400 | 5000 | 37 | 23 | 14 | - | - | 10 | 84 | 1.68 ± 0.25 | |
| ZnO-Alg/NCMs 400 | 5000 | 33 | 22 | 19 | - | - | 17 | 91 | 1.82 ± 0.33 | |
| AL 400 + MMC | 5000 | 95 | 112 | 72 | 6 | 2 | 145 | 432 | 8.64 ± 0.43 ab | 39.15 |
| ZnO-Alg/NCMs 400 + MMC | 5000 | 88 | 109 | 68 | 5 | 1 | 87 | 358 | 7.16 ± 0.7 ab | 49.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamouda, R.A.; Salman, A.S.; Alharbi, A.A.; Alhasani, R.H.; Elshamy, M.M. Assessment of the Antigenotoxic Effects of Alginate and ZnO/Alginate–Nanocomposites Extracted from Brown Alga Fucus vesiculosus in Mice. Polymers 2021, 13, 3839. https://doi.org/10.3390/polym13213839
Hamouda RA, Salman AS, Alharbi AA, Alhasani RH, Elshamy MM. Assessment of the Antigenotoxic Effects of Alginate and ZnO/Alginate–Nanocomposites Extracted from Brown Alga Fucus vesiculosus in Mice. Polymers. 2021; 13(21):3839. https://doi.org/10.3390/polym13213839
Chicago/Turabian StyleHamouda, Ragaa A., Asmaa S. Salman, Asrar A. Alharbi, Reem Hasaballah Alhasani, and Maha M. Elshamy. 2021. "Assessment of the Antigenotoxic Effects of Alginate and ZnO/Alginate–Nanocomposites Extracted from Brown Alga Fucus vesiculosus in Mice" Polymers 13, no. 21: 3839. https://doi.org/10.3390/polym13213839