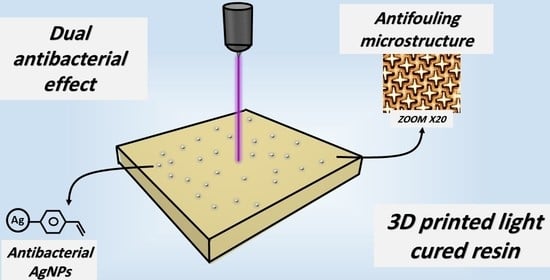
Printable Resin Modified by Grafted Silver Nanoparticles for Preparation of Antifouling Microstructures with Antibacterial Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Silver Nanoparticles Preparation
2.3. Characterization Methods
2.3.1. Physical and Chemical Characterization
2.3.2. Material Functionality and Its Antibacterial Properties
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rafique, M.; Sadaf, I.; Tahir, M.B.; Nabi, G.; Iqbal, T.; Sughra, K. Novel and facile synthesis of silver nanoparticles using Albizia procera leaf extract for dye degradation and antibacterial applications. Mater. Sci. Eng. C 2019, 99, 1313–1324. [Google Scholar] [CrossRef]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L.; et al. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469–1487. [Google Scholar] [CrossRef] [Green Version]
- Sofi, H.S.; Akram, T.; Tamboli, A.H.; Majeed, A.; Shabir, N.; Sheikh, F.A. Novel lavender oil and silver nanoparticles simultaneously loaded onto polyurethane nanofibers for wound-healing applications. Int. J. Pharm. 2019, 569, 118590. [Google Scholar] [CrossRef]
- Kaur, A.; Goyal, D.; Kumar, R. Surfactant mediated interaction of vancomycin with silver nanoparticles. Appl. Surf. Sci. 2018, 449, 23–30. [Google Scholar] [CrossRef]
- Elashnikov, R.; Slepička, P.; Rimpelova, S.; Ulbrich, P.; Švorčík, V.; Lyutakov, O. Temperature-responsive PLLA/PNIPAM nanofibers for switchable release. Mater. Sci. Eng. C 2017, 72, 293–300. [Google Scholar] [CrossRef]
- Baldino, L.; Aragón, J.; Mendoza, G.; Irusta, S.; Cardea, S.; Reverchon, E. Production, characterization and testing of antibacterial PVA membranes loaded with HA-Ag3 PO4 nanoparticles, produced by SC-CO2 phase inversion. J. Chem. Technol. Biotechnol. 2018, 94, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Celebioglu, A.; Topuz, F.; Yildiz, Z.I.; Uyar, T. One-step green synthesis of antibacterial silver nanoparticles embedded in electrospun cyclodextrin nanofibers. Carbohydr. Polym. 2018, 207, 471–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Carretero, S.; Nybom, R.; Richter-Dahlfors, A. Electroenhanced Antimicrobial Coating Based on Conjugated Polymers with Covalently Coupled Silver Nanoparticles Prevents Staphylococcus aureus Biofilm Formation. Adv. Heal. Mater. 2017, 6, 1700435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyutakov, O.; Goncharova, I.; Rimpelova, S.; Kolarova, K.; Svanda, J.; Svorcik, V. Silver release and antimicrobial properties of PMMA films doped with silver ions, nano-particles and complexes. Mater. Sci. Eng. C 2015, 49, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Yang, J.; Lou, Y.; Rahman, O.U.; Li, Z.; Ding, X.; Gao, J.; Du, C.; Zhang, D. Mussel-inspired superhydrophilic surface with enhanced antimicrobial properties under immersed and atmospheric conditions. Appl. Surf. Sci. 2018, 465, 267–278. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. One-Step Synthesis of Silver Nanoparticles Embedded Polyurethane Nano-Fiber/Net Structured Membrane as an Effective Antibacterial Medium. Polymers 2019, 11, 1185. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-C.; Shiao, J.-H.; Tsai, T.-L.; Jiang, D.-H.; Chen, L.-C.; Chang, C.-H.; Lin, B.-H.; Lin, J.H.; Kuo, C.C. Multiple Scattering from Electrospun Nanofibers with Embedded Silver Nanoparticles of Tunable Shape for Random Lasers and White-Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2020, 12, 2783–2792. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-Y.; Cheng, C.-H.; Hung, S.-K. 3D-Printed Linear Positioner with Micrometer Accuracy. MATEC Web Conf. 2017, 95, 10005. [Google Scholar] [CrossRef] [Green Version]
- Ertugrul, I. The Fabrication of Micro Beam from Photopolymer by Digital Light Processing 3D Printing Technology. Micromachines 2020, 11, 518. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.; Elashnikov, R.; Guselnikova, O.; Postnikov, P.; Kolska, Z.; Lyutakov, O.; Švorčík, V. Reversible wettability switching of piezo-responsive nanostructured polymer fibers by electric field. Chem. Pap. 2020, 75, 191–196. [Google Scholar] [CrossRef]
- Sikder, M.; Lead, J.R.; Chandler, G.T.; Baalousha, M. A rapid approach for measuring silver nanoparticle concentration and dissolution in seawater by UV-Vis, Sci. Total Environ. 2018, 618, 597–607. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 19, 4855–4861. [Google Scholar] [CrossRef]
- Idriss, H.; Guselnikova, O.; Postnikov, P.; Kolská, Z.; Haušild, P.; Lyutakov, O.; Švorčík, V. Polymer icephobic surface by graphite coating and chemical grafting with diazonium salts. Surf. Interfaces 2021, 25, 101226. [Google Scholar] [CrossRef]
- Oliver, W.; Pharr, G. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Hoboken, NJ, USA, 2004; pp. 154–196. [Google Scholar]
- Kolarova, K.; Vosmanská, V.; Rimpelova, S.; Švorčík, V. Effect of plasma treatment on cellulose fiber. Cellulose 2013, 20, 953–961. [Google Scholar] [CrossRef]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef]
- Pišlová, M.; Kolářová, K.; Vokatá, B.; Brož, A.; Ulbrich, P.; Bačáková, L.; Kolská, Z.; Švorčík, V. A new way to prepare gold nanoparticles by sputtering—Sterilization, stability and other properties. Mater. Sci. Eng. C 2020, 115, 111087. [Google Scholar] [CrossRef] [PubMed]
- Polívková, M.; Štrublová, V.; Hubáček, T.; Rimpelová, S.; Švorčík, V.; Siegel, J. Surface characterization and antibacterial response of silver nanowire arrays supported on laser-treated polyethylene naphthalate. Mater. Sci. Eng. C 2017, 72, 512–518. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Vasudevan, R.; Pappas, D.D.; A Weiss, C.; Hendrix, S.H.; Baney, R.H. Efficacy of non-toxic surfaces to reduce bioadhesion in terrestrial gastropods. Pest Manag. Sci. 2011, 67, 318–327. [Google Scholar] [CrossRef]
- Vasudevan, R.; Kennedy, A.J.; Merritt, M.; Crocker, F.H.; Baney, R.H. Microscale patterned surfaces reduce bacterial fouling-microscopic and theoretical analysis. Colloids Surfaces B Biointerfaces 2014, 117, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Děkanovský, L.; Elashnikov, R.; Kubiková, M.; Vokatá, B.; Švorčík, V.; Lyutakov, O. Dual-Action Flexible Antimicrobial Material: Switchable Self-Cleaning, Antifouling, and Smart Drug Release. Adv. Funct. Mater. 2019, 29, 1901880. [Google Scholar] [CrossRef]
- Wen, X.; Almousa, R.; Anderson, G.G.; Xie, D. Developing a novel antibacterial dental resin composite with improved properties. J. Compos. Mater. 2018, 53, 3085–3092. [Google Scholar] [CrossRef]






| Sample | H (MPa) | E (GPa) |
|---|---|---|
| Pristine | 198.3 ± 8.4 | 4.0 ± 0.2 |
| Modified | 185.0 ± 17.3 | 3.5 ± 0.2 |
| Modified after 1 week | 201.9 ± 14.5 | 3.8 ± 0.1 |
| Modified after 1 month | 194.9 ± 16.9 | 3.8 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idriss, H.; Elashnikov, R.; Rimpelová, S.; Vokatá, B.; Haušild, P.; Kolská, Z.; Lyukatov, O.; Švorčík, V. Printable Resin Modified by Grafted Silver Nanoparticles for Preparation of Antifouling Microstructures with Antibacterial Effect. Polymers 2021, 13, 3838. https://doi.org/10.3390/polym13213838
Idriss H, Elashnikov R, Rimpelová S, Vokatá B, Haušild P, Kolská Z, Lyukatov O, Švorčík V. Printable Resin Modified by Grafted Silver Nanoparticles for Preparation of Antifouling Microstructures with Antibacterial Effect. Polymers. 2021; 13(21):3838. https://doi.org/10.3390/polym13213838
Chicago/Turabian StyleIdriss, Hazem, Roman Elashnikov, Silvie Rimpelová, Barbora Vokatá, Petr Haušild, Zdeňka Kolská, Oleksiy Lyukatov, and Václav Švorčík. 2021. "Printable Resin Modified by Grafted Silver Nanoparticles for Preparation of Antifouling Microstructures with Antibacterial Effect" Polymers 13, no. 21: 3838. https://doi.org/10.3390/polym13213838