A Study of Compressibility, Compactability and Mucoadhesivity of Tableting Materials for Matrix Systems Based on Chitosan
Abstract
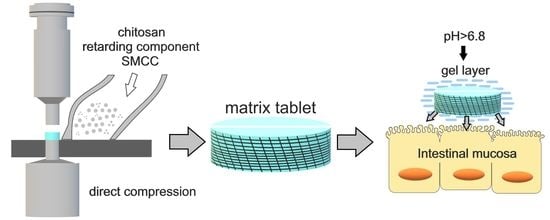
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Tableting Materials
2.3. Preparation of Tablets and Energy Evaluation of Compression Process
2.4. Evaluation of Compactability and Lubricant Sensitivity
2.5. Rheological Testing
2.6. Mucoadhesion Testing
2.7. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Compressibility
3.2. Evaluation of Compactability and Lubricant Sensitivity
3.3. Rheological and Mucoadhesion Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yilmaz, N.D.; Çilgi, G.K.; Yilmaz, K. Natural Polysaccharides as Pharmaceutical Excipients. In Handbook of Polymers for Pharmaceutical Technologies; Thakur, V.K., Thakur, M.K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 483–516. [Google Scholar]
- Vandamme, T. The Use of Polysaccharides to Target Drugs to the Colon. Carbohydr. Polym. 2002, 48, 219–231. [Google Scholar] [CrossRef]
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract—Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef]
- Jain, V.; Shukla, N.; Mahajan, S.C. Polysaccharides in Colon Specific Drug Delivery. J. Transl. Sci. 2015, 1, 3–11. [Google Scholar]
- Khvostov, M.V.; Tolstikova, T.G.; Borisov, S.A.; Dushkin, A.V. Application of Natural Polysaccharides in Pharmaceutics. Russ. J. Bioorg. Chem. 2019, 45, 438–450. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Pisani, A.; Gatto, F.; Bardi, G. Natural Polysaccharide Nanomaterials: An Overview of Their Immunological Properties. Int. J. Mol. Sci. 2019, 20, 5092. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanovska, A.; Husak, Y.; Korniienko, V.; Holubnycha, V.; Mishchenko, O.; Banasiuk, R.; Radwan-Pragłowska, J.; Piątkowski, M.; Janus, Ł.; Pogorielov, M. Development, Characterization and Antimicrobial Properties of Silver Nanoparticles Loaded Chitosan-Alginate Sponges for Biomedical Application. J. Mater. Res. 2021, 36, 3267–3277. [Google Scholar] [CrossRef]
- Deineka, V.; Sulaieva, O.; Pernakov, M.; Korniienko, V.; Husak, Y.; Yanovska, A.; Yusupova, A.; Tkachenko, Y.; Kalinkevich, O.; Zlatska, A.; et al. Hemostatic and Tissue Regeneration Performance of Novel Electrospun Chitosan-Based Materials. Biomedicines 2021, 9, 588. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical Applications of Chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, M.K.; Jain, S.K. Polysaccharides for Colon Targeted Drug Delivery. Drug Deliv. 2004, 11, 129–148. [Google Scholar] [CrossRef] [Green Version]
- Safdar, R.; Omar, A.A.; Arunagiri, A.; Regupathi, I.; Thanabalan, M. Potential of Chitosan and Its Derivatives for Controlled Drug Release Applications—A Review. J. Drug Deliv. Sci. Technol. 2019, 49, 642–659. [Google Scholar] [CrossRef]
- Rabiskova, M.; Kubova-Dvorackova, K.; Kofronva, L. Stability Testing of Alginate-Chitosan Films. Ceska Slov. Farm. 2012, 61, 26–33. [Google Scholar]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial Activity of Chitosan and Its Derivatives and Their Interaction Mechanism with Bacteria: Current State and Perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive Polymeric Platforms for Controlled Drug Delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef]
- Hejazi, R.; Amiji, M. Chitosan-Based Gastrointestinal Delivery Systems. J. Control. Release 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Dobrynin, A.; Rubinstein, M. Theory of Polyelectrolytes in Solutions and at Surfaces. Prog. Polym. Sci. 2005, 30, 1049–1118. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.; Felt, O.; Gurny, R. Structure and Interactions in Chitosan Hydrogels Formed by Complexation or Aggregation for Biomedical Applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Shao, Y.; Ni, R.; Zhang, T.; Mao, S. Drug Release Characteristics from Chitosan—Alginate Matrix Tablets Based on the Theory of Self-Assembled Film. Int. J. Pharm. 2013, 450, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Badwan, A.; Rashid, I.; Omari, M.; Darras, F. Chitin and Chitosan as Direct Compression Excipients in Pharmaceutical Applications. Mar. Drugs 2015, 13, 1519–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, V.; Heinamaki, J.; Antikainen, O.; Revoredo, O.; Colarte, A.; Nieto, O.; Yliruusi, J. Direct Compression Properties of Chitin and Chitosan. Eur. J. Pharm. Biopharm. 2008, 69, 964–968. [Google Scholar] [CrossRef]
- Rojas, J.; Hernandez, C.; Trujillo, D. Effect of the Alkaline Treatment Conditions on the Tableting Performance of Chitin Obtained from Shrimp Heads. Afr. J. Pharm. Pharmacol. 2014, 8, 211–219. [Google Scholar]
- Aucamp, M.E. Assessment of the Tableting Properties of Chitosan through Wet Granulation and Direct Compression Formulations. Master’s Thesis, North West University, Potchefstroom, South Africa, 2004. [Google Scholar]
- Ragnarsson, G. Force-Displacement and Network Measurements. In Pharmaceutical Powder Compaction Technology; Alderborn, G., Nyström, C., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 77–96. [Google Scholar]
- Stamm, A.; Mathis, C. Compressibility of Solid Excipients for Direct Compression. Acta Pharm. Technol. 1976, 22, 7–16. [Google Scholar]
- Fell, J.T.; Newton, J.M. Determination of Tablet Strength by the Diametral-Compression Test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef]
- Bos, C.E.; Bolhuis, G.K.; van Doorne, H.; Lerk, C.F. Native Starch in Tablet Formulations: Properties on Compaction. Pharm. Weekbl. 1987, 9, 274–282. [Google Scholar] [CrossRef]
- van Veen, B.; Bolhuis, G.K.; Wu, Y.S.; Zuurman, K.; Frijlink, H.W. Compaction Mechanism and Tablet Strength of Unlubricated and Lubricated (Silicified) Microcrystalline Cellulose. Eur. J. Pharm. Biopharm. 2005, 59, 133–138. [Google Scholar] [CrossRef]
- Mužíková, J.; Nováková, P. A Study of the Properties of Compacts from Silicified Microcrystalline Celluloses. Drug Dev. Ind. Pharm. 2007, 33, 775–781. [Google Scholar] [CrossRef]
- Moreton, R.C. Cellulose Silicified Microcrystalline. In Handbook of Pharmaceutical Excipients; Sheskey, P.J., Cook, W.G., Cable, C.G., Eds.; Pharmaceutical Press London and American Pharmaceutical Association: Washington, DC, USA, 2017; pp. 204–206. [Google Scholar]
- Li, J.; Wu, Y. Lubricants in Pharmaceutical Solid Dosage Forms. Lubricants 2014, 2, 21–43. [Google Scholar] [CrossRef]
- Bolhuis, G.K.; Lerk, C.F. Ordered Mixing with Lubricant and Glidant in Tableting Mixtures. J. Pharm. Pharmacol. 2011, 33, 790. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.F. Weak and Strong Gels and the Emergence of the Amorphous Solid State. Gels 2018, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rencber, S.; Cheaburu-Yilmaz, C.N.; Köse, F.A.; Yaprak Karavana, S.; Yilmaz, O. Preparation and Characterization of Alginate and Chitosan IPC Based Gel Formulation for Mucosal Application. Cellul. Chem. Technol. 2019, 53, 655–665. [Google Scholar] [CrossRef]
- Bappaditya, C.; Nursazreen, A.; Pinaki, S.; Uttam, K.M. Mucoadhesive Polymers and Their Mode of Action: A Recent Update. J. Appl. Pharm. Sci. 2017, 7, 195–203. [Google Scholar]
- Cazorla-Luna, R.; Martín-Illana, A.; Notario-Pérez, F.; Ruiz-Caro, R.; Veiga, M.-D. Naturally Occurring Polyelectrolytes and Their Use for the Development of Complex-Based Mucoadhesive Drug Delivery Systems: An Overview. Polymers 2021, 13, 2241. [Google Scholar] [CrossRef] [PubMed]



| Tableting Material | CH (%) | SA (%) | HPMC100M (%) | Mgst (%) |
|---|---|---|---|---|
| F1 | 100 | - | - | - |
| F2 | 70 | 30 | - | - |
| F3 | 60 | 40 | - | - |
| F4 | 50 | 50 | - | - |
| F1L | 99 | - | - | 1 |
| F2L | 69 | 30 | - | 1 |
| F3L | 59 | 40 | - | 1 |
| F4L | 49 | 50 | - | 1 |
| F5 | 70 | 15 | 15 | - |
| F6 | 60 | 20 | 20 | - |
| F7 | 50 | 25 | 25 | - |
| F5L | 69 | 15 | 15 | 1 |
| F6L | 59 | 20 | 20 | 1 |
| F7L | 49 | 25 | 25 | 1 |
| Tableting Material | CH + P90 3:1 (%) | SA (%) | HPMC100M (%) | Mgst (%) |
|---|---|---|---|---|
| FP1 | 100 | - | - | - |
| FP2 | 70 | 30 | - | - |
| FP3 | 60 | 40 | - | - |
| FP4 | 50 | 50 | - | - |
| FP1L | 99 | - | - | 1 |
| FP2L | 69 | 30 | - | 1 |
| FP3L | 59 | 40 | - | 1 |
| FP4L | 49 | 50 | - | 1 |
| FP5 | 70 | 15 | 15 | - |
| FP6 | 60 | 20 | 20 | - |
| FP7 | 50 | 25 | 25 | - |
| FP5L | 69 | 15 | 15 | 1 |
| FP6L | 59 | 20 | 20 | 1 |
| FP7L | 49 | 25 | 25 | 1 |
| Tableting Material | Emax ± SD (J) | E1 ± SD (J) | E2 ± SD (J) | E3 ± SD (J) | Pl ± SD (J) |
|---|---|---|---|---|---|
| F1 | 6.31 ± 0.44 | 4.07 ± 0.44 | 1.76 ± 0.05 | 0.48 ± 0.01 | 78.66 ± 0.52 |
| F2 | 4.50 ± 0.37 | 2.34 ± 0.26 | 1.38 ± 0.12 | 0.47 ± 0.01 | 78.05 ± 0.91 |
| F3 | 3.84 ± 0.39 | 1.84 ± 0.20 | 1.54 ± 0.18 | 0.46 ± 0.01 | 77.03 ± 1.78 |
| F4 | 4.07 ± 0.37 | 1.90 ± 0.20 | 1.71 ± 0.17 | 0.46 ± 0.01 | 78.68 ± 1.25 |
| F1L | 5.21 ± 0.25 | 3.09 ± 0.16 | 1.62 ± 0.08 | 0.49 ± 0.02 | 76.63 ± 0.62 |
| F2L | 4.05 ± 0.14 | 2.11 ± 0.10 | 1.47 ± 0.05 | 0.47 ± 0.00 | 75.82 ± 0.54 |
| F3L | 3.60 ± 0.17 | 1.73 ± 0.14 | 1.41 ± 0.04 | 0.46 ± 0.00 | 75.42 ± 0.53 |
| F4L | 3.43 ± 0.23 | 1.58 ± 0.18 | 1.39 ± 0.05 | 0.46 ± 0.01 | 75.31 ± 0.52 |
| F5 | 5.77 ± 0.32 | 3.63 ± 0.30 | 1.68 ± 0.05 | 0.45 ± 0.01 | 78.73 ± 0.45 |
| F6 | 5.72 ± 0.44 | 3.63 ± 0.43 | 1.63 ± 0.02 | 0.46 ± 0.00 | 78.10 ± 0.28 |
| F7 | 5.60 ± 0.33 | 3.53 ± 0.31 | 1.62 ± 0.03 | 0.45 ± 0.01 | 78.13 ± 0.28 |
| F5L | 5.88 ± 0.17 | 3.87 ± 0.14 | 1.55 ± 0.05 | 0.46 ± 0.01 | 77.24 ± 0.32 |
| F6L | 5.73 ± 0.19 | 3.66 ± 0.17 | 1.60 ± 0.05 | 0.46 ± 0.01 | 77.54 ± 0.36 |
| F7L | 4.99 ± 0.31 | 3.03 ± 0.30 | 1.50 ± 0.03 | 0.46 ± 0.01 | 76.76 ± 0.25 |
| Tableting Material | Emax ± SD (J) | E1 ± SD (J) | E2 ± SD (J) | E3 ± SD (J) | Pl ± SD (J) |
|---|---|---|---|---|---|
| FP1 | 7.44 ± 0.13 | 4.98 ± 0.11 | 1.99 ± 0.04 | 0.47 ± 0.01 | 80.88 ± 0.22 |
| FP2 | 5.78 ± 0.14 | 3.49 ± 0.13 | 1.78 ± 0.01 | 0.45 ± 0.00 | 79.69 ± 0.18 |
| FP3 | 5.46 ± 0.08 | 3.28 ± 0.08 | 1.73 ± 0.01 | 0.45 ± 0.00 | 79.38 ± 0.14 |
| FP4 | 5.04 ± 0.12 | 2.91 ± 0.11 | 1.68 ± 0.01 | 0.45 ± 0.01 | 79.00 ± 0.15 |
| FP1L | 6.17 ± 0.05 | 3.84 ± 0.05 | 1.91 ± 0.02 | 0.43 ± 0.00 | 81.70 ± 0.13 |
| FP2L | 5.43 ± 0.14 | 3.31 ± 0.13 | 1.66 ± 0.01 | 0.46 ± 0.00 | 78.18 ± 0.15 |
| FP3L | 4.97 ± 0.09 | 2.90 ± 0.08 | 1.62 ± 0.01 | 0.46 ± 0.00 | 77.99 ± 0.14 |
| FP4L | 4.46 ± 0.09 | 2.46 ± 0.08 | 1.55 ± 0.01 | 0.46 ± 0.02 | 77.22 ± 0.24 |
| FP5 | 6.16 ± 0.22 | 3.89 ± 0.23 | 1.83 ± 0.02 | 0.45 ± 0.01 | 80.40 ± 0.25 |
| FP6 | 5.98 ± 0.30 | 3.80 ± 0.31 | 1.75 ± 0.03 | 0.43 ± 0.01 | 80.26 ± 0.43 |
| FP7 | 6.09 ± 0.20 | 3.92 ± 0.20 | 1.74 ± 0.02 | 0.43 ± 0.01 | 80.36 ± 0.21 |
| FP5L | 6.25 ± 0.18 | 4.07 ± 0.18 | 1.74 ± 0.02 | 0.44 ± 0.00 | 79.84 ± 0.25 |
| FP6L | 6.16 ± 0.12 | 4.03 ± 0.11 | 1.68 ± 0.01 | 0.44 ± 0.00 | 79.15 ± 0.22 |
| FP7L | 5.93 ± 0.18 | 3.86 ± 0.17 | 1.64 ± 0.03 | 0.44 ± 0.00 | 78.94 ± 0.30 |
| Tableting Material | TS ± SD (MPa) | LSR ± SD | Tableting Material | TS ± SD (MPa) | LSR ± SD |
|---|---|---|---|---|---|
| F1 | 1.212 ± 0.072 | 0.40 ± 0.02 | FP1 | 1.909 ± 0.160 | 0.01 ± 0.03 |
| F2 | 0.682 ± 0.035 | 0.30 ± 0.02 | FP2 | 1.312 ± 0.068 | 0.26 ± 0.02 |
| F3 | 0.554 ± 0.054 | 0.27 ± 0.03 | FP3 | 1.302 ± 0.079 | 0.44 ± 0.01 |
| F4 | 0.484 ± 0.053 | 0.29 ± 0.05 | FP4 | 0.999 ± 0.077 | 0.53 ± 0.01 |
| F1L | 0.730 ± 0.047 | - | FP1L | 1.885 ± 0.036 | - |
| F2L | 0.476 ± 0.037 | - | FP2L | 0.973 ± 0.051 | - |
| F3L | 0.407 ± 0.020 | - | FP3L | 0.732 ± 0.029 | - |
| F4L | 0.341 ± 0.059 | - | FP4L | 0.471 ± 0.024 | - |
| F5 | 1.873 ± 0.117 | 0.16 ± 0.02 | FP8 | 2.756 ± 0.118 | 0.10 ± 0.01 |
| F6 | 1.977 ± 0.141 | 0.24 ± 0.02 | FP9 | 2.631 ± 0.161 | 0.15 ± 0.03 |
| F7 | 2.016 ± 0.061 | 0.30 ± 0.01 | FP10 | 2.457 ± 0.054 | 0.17 ± 0.02 |
| F5L | 1.571 ± 0.062 | - | FP8L | 2.494 ± 0.030 | - |
| F6L | 1.510 ± 0.064 | - | FP9L | 2.240 ± 0.159 | - |
| F7L | 1.404 ± 0.057 | - | FP10L | 2.030 ± 0.124 | - |
| Retarding Component | n Values for CH | n Values for CH/P90 |
|---|---|---|
| SA 30% | 0.5847 ± 0.005 | 0.5356 ± 0.047 |
| SA 40% | 0.5608 ± 0.012 | 0.5305 ± 0.011 |
| SA 50% | 0.5050 ± 0.007 | 0.4937 ± 0.006 |
| SA/HPMC100M 30% | 0.4665 ± 0.014 | 0.4950 ± 0.011 |
| SA/HPMC100M 40% | 0.4702 ± 0.019 | 0.4867 ± 0.018 |
| SA/HPMC100M 50% | 0.4642 ± 0.002 | 0.4389 ± 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzikova, J.; Snejdrova, E.; Martiska, J.; Doubkova, B.; Veris, A. A Study of Compressibility, Compactability and Mucoadhesivity of Tableting Materials for Matrix Systems Based on Chitosan. Polymers 2021, 13, 3636. https://doi.org/10.3390/polym13213636
Muzikova J, Snejdrova E, Martiska J, Doubkova B, Veris A. A Study of Compressibility, Compactability and Mucoadhesivity of Tableting Materials for Matrix Systems Based on Chitosan. Polymers. 2021; 13(21):3636. https://doi.org/10.3390/polym13213636
Chicago/Turabian StyleMuzikova, Jitka, Eva Snejdrova, Juraj Martiska, Bara Doubkova, and Andrea Veris. 2021. "A Study of Compressibility, Compactability and Mucoadhesivity of Tableting Materials for Matrix Systems Based on Chitosan" Polymers 13, no. 21: 3636. https://doi.org/10.3390/polym13213636