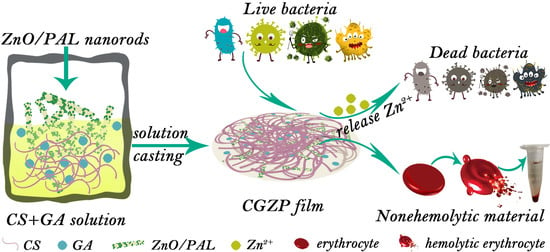
Synergistic Effect of Glycyrrhizic Acid and ZnO/Palygorskite on Improving Chitosan-Based Films and Their Potential Application in Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CG and CGZP Films
2.3. Characterizations of CG and CGZP Films
2.4. Mechanical Properties of CG and CGZP Films
2.5. Physicochemical Properties of CG and CGZP Films
2.6. Light Transmittance and Opacity of CG and CGZP Films
2.7. Antibacterial Activity of CG and CGZP Films
2.8. Hemolysis Experiment of CG and CGZP Films
2.9. Statistical Analysis
3. Results
3.1. Characterizations of CG and CGZP Films
3.2. Mechanical Properties of CG and CGZP Films
3.3. Physicochemical Properties of CG and CGZP Films
3.4. Light Transmittance and Opacity of CG and CGZP Films
3.5. Antibacterial Activity of CG and CGZP Films
3.6. Hemocompatibility of CG and CGZP Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, M.; Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Yoo, J.W. Chitosan-based nitric oxide-releasing dressing for anti-biofilm and in vivo healing activities in MRSA biofilm-infected wounds. Int. J. Biol. Macromol. 2020, 142, 680–692. [Google Scholar] [CrossRef]
- Flokas, M.E.; Detsis, M.; Alevizakos, M.; Mylonakis, E. Prevalence of ESBL—Producing Enterobacteriaceae in paediatric urinary tract infections: A systematic review and meta-analysis. J. Infect. 2016, 73, 547–557. [Google Scholar] [CrossRef]
- Wright, G.D. Solving the antibiotic crisis. ACS Infect. Dis. 2015, 1, 80–84. [Google Scholar] [CrossRef]
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, P.; Ramalingam, B.; Das, S.K. Fabrication of chitosan-reinforced multifunctional graphene nanocomposite as antibacterial scaffolds for hemorrhage control and wound-healing application. ACS Biomater. Sci. Eng. 2020, 6, 5911–5929. [Google Scholar] [CrossRef]
- Shivakumar, P.; Gupta, M.S.; Jayakumar, R.; Gowda, D.V. Prospection of chitosan and its derivatives in wound healing: Proof of patent analysis (2010–2020). Int. J. Biol. Macromol. 2021, 184, 701–712. [Google Scholar] [CrossRef]
- M Ways, T.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Alamry, K.A. Recent advances of emerging green chitosan-based biomaterials with potential biomedical applications: A review. Carbohydr. Res. 2021, 506, 108368. [Google Scholar] [CrossRef]
- Doench, I.; Torres-Ramos, M.E.W.; Montembault, A.; de Oliveira, P.N.; Halimi, C.; Viguier, E.; Heux, L.; Siadous, R.; Thiré, R.M.S.M.; Osorio-Madrazo, A. Injectable and gellable chitosan formulations filled with cellulose nanofibers for intervertebral disc tissue engineering. Polymers 2018, 10, 1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.; Ji, Z.; Xu, M.; Liu, C.; Ye, X.; Zhang, W.; Li, S.; Wang, D.; Zhang, W.; Chen, J.; et al. Microspheres of carboxymethyl chitosan, sodium alginate, and collagen as a hemostatic agent in vivo. ACS Biomater. Sci. Eng. 2018, 4, 2541–2551. [Google Scholar] [CrossRef]
- Irimia, T.; Ghica, M.V.; Popa, L.; Anuţa, V.; Arsene, A.L.; Dinu-Pîrvu, C.E. Strategies for improving ocular drug bioavailability and cornealwound healing with chitosan-based delivery systems. Polymers 2018, 10, 1221. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Xiao, H.; Seidi, F.; Jin, Y. Natural polymer-based antimicrobial hydrogels without synthetic antibiotics as wound dressings. Biomacromolecules 2020, 21, 2983–3006. [Google Scholar] [CrossRef]
- Wittaya-areekul, S.; Prahsarn, C. Development and in vitro evaluation of chitosan-polysaccharides composite wound dressings. Int. J. Pharm. 2006, 313, 123–128. [Google Scholar] [CrossRef]
- Zeng, A.; Wang, Y.; Li, D.; Guo, J.; Chen, Q. Preparation and antibacterial properties of polycaprolactone/quaternized chitosan blends. Chin. J. Chem. Eng. 2021, 32, 462–471. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, W.; Ding, J.; Lu, Y.; Xu, J.; Wang, A. An upgraded and universal strategy to reinforce chitosan/polyvinylpyrrolidone film by incorporating active silica nanorods derived from natural palygorskite. Int. J. Biol. Macromol. 2020, 165, 1276–1285. [Google Scholar] [CrossRef]
- Mu, B.; Wang, A. One-pot fabrication of multifunctional superparamagnetic attapulgite/Fe3O4/polyaniline nanocomposites served as an adsorbent and catalyst support. J. Mater. Chem. A 2015, 3, 281–289. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A. Recent progress in dispersion of palygorskite crystal bundles for nanocomposites. Appl. Clay Sci. 2016, 119, 18–30. [Google Scholar] [CrossRef]
- Ma, B.; Sun, B.; Huang, Y.; Chen, C.; Sun, D. Facile synthesis of Cu nanoparticles encapsulated into carbonized bacterial cellulose with excellent oxidation resistance and stability. Colloid Surf. A Physicochem. Eng. Asp. 2020, 590, 124462. [Google Scholar] [CrossRef]
- Khawaja, H.; Zahir, E.; Asghar, M.A.; Asghar, M.A. Graphene oxide, chitosan and silver nanocomposite as a highly effective antibacterial agent against pathogenic strains. Colloid Surf. A Physicochem. Eng. Asp. 2018, 555, 246–255. [Google Scholar] [CrossRef]
- Aradmehr, A.; Javanbakht, V. A novel biofilm based on lignocellulosic compounds and chitosan modified with silver nanoparticles with multifunctional properties: Synthesis and characterization. Colloid Surf. A Physicochem. Eng. Asp. 2020, 600, 124952. [Google Scholar] [CrossRef]
- Rakhshaei, R.; Namazi, H. A potential bioactive wound dressing based on carboxymethyl cellulose/ZnO impregnated MCM-41 nanocomposite hydrogel. Mater. Sci. Eng. C 2017, 73, 456–464. [Google Scholar] [CrossRef]
- Balaure, P.C.; Holban, A.M.; Grumezescu, A.M.; Mogoşanu, G.D.; Bălşeanu, T.A.; Stan, M.S.; Dinischiotu, A.; Volceanov, A.; Mogoantă, L. In vitro and in vivo studies of novel fabricated bioactive dressings based on collagen and zinc oxide 3D scaffolds. Int. J. Pharm. 2019, 557, 199–207. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Acharya, B.; Korber, D.R. Antimicrobial biodegradable food packaging based on chitosan and metal/metal-oxide bio-nanocomposites: A review. Polymers 2021, 13, 2790. [Google Scholar] [CrossRef]
- Hui, A.; Yan, R.; Wang, W.; Wang, Q.; Zhou, Y.; Wang, A. Incorporation of quaternary ammonium chitooligosaccharides on ZnO/palygorskite nanocomposites for enhancing antibacterial activities. Carbohydr. Polym. 2020, 247, 116685. [Google Scholar] [CrossRef]
- Hui, A.; Yan, R.; Mu, B.; Kang, Y.; Zhou, Y.; Wang, A. Preparation and antibacterial activity of ZnO/palygorskite nanocomposites using different types of surfactants. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3808–3817. [Google Scholar] [CrossRef]
- Sahin, F.; Oznurhan, F. Antibacterial efficacy and remineralization capacity of glycyrrhizic acid added casein phosphopeptide-amorphous calcium phosphate. Microsc. Res. Tech. 2020, 83, 744–754. [Google Scholar] [CrossRef]
- Duran, G.G.; Duran, N.; Aslan, H.; Jenedi, K.; Mert, A.; Boycigil, B.C.I. Antibacterial activity of glycyrrhizic acid against multi drug resistant bacteria and fungus. In Proceedings of the 5th International Conference on Advanced Materials and Systems, Bucharest, Romania, 23–25 October 2014; pp. 189–194. [Google Scholar]
- Zhao, X.; Zhang, H.; Gao, Y.; Lin, Y.; Hu, J. A simple injectable moldable hydrogel assembled from natural Glycyrrhizic acid with inherent antibacterial activity. ACS Appl. Bio Mater. 2019, 3, 648–653. [Google Scholar] [CrossRef] [Green Version]
- Jamróz, E.; Khachatryan, G.; Kopel, P.; Juszczak, L.; Kawecka, A.; Krzyściak, P.; Kucharek, M.; Bębenek, Z.; Zimowska, M. Furcellaran nanocomposite films: The effect of nanofillers on the structural, thermal, mechanical and antimicrobial properties of biopolymer films. Carbohydr. Polym. 2020, 240, 116244. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, X.; Li, X.; Li, Y.; Zhao, C.; Jiang, Z. Enhancing structural stability and pervaporation performance of composite membranes by coating gelatin onto hydrophilically modified support layer. Chin. J. Chem. Eng. 2014, 22, 19–27. [Google Scholar] [CrossRef]
- Li, X.; Ma, M.; Ahn, D.U.; Huang, X. Preparation and characterization of novel eggshell membrane-chitosan blend films for potential wound-care dressing: From waste to medicinal products. Int. J. Biol. Macromol. 2019, 123, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Trusca, R.D.; Andronescu, E.; Holban, A.M. Biodegradable alginate films with ZnO nanoparticles and citronella essential oil—A novel antimicrobial structure. Pharmaceutics 2021, 13, 1020. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeong, D.; Kanmani, P. Study on physical and mechanical properties of the biopolymer/silver based active nanocomposite films with antimicrobial activity. Carbohydr. Polym. 2019, 224, 115159. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, T.; Wang, C.; Wang, Z.; Yang, Y.; Li, P.; Cai, R.; Sun, M.; Yuan, H.; Nie, L. Synthesis and characterization of silver nanoparticles-doped hydroxyapatite/alginate microparticles with promising cytocompatibility and antibacterial properties. Colloid Surf. A Physicochem. Eng. Asp. 2020, 585, 124081. [Google Scholar] [CrossRef]
- Silva, S.M.L.; Braga, C.R.C.; Fook, M.V.L.; Raposo, C.M.O.; Carvalho, L.H.; Canedo, E.L. Application of infrared spectroscopy to analysis of chitosan/clay nanocomposites. Infrared Spectrosc. Mater. Sci. Eng. Technol. 2012, 2, 43–62. [Google Scholar] [CrossRef]
- Yang, J.; Kwon, G.J.; Hwang, K.; Kim, D.Y. Cellulose-chitosan antibacterial composite films prepared from libr solution. Polymers 2018, 10, 1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, C.; Kóczán, Z.; Börcsök, Z.; Halász, K.; Pásztory, Z. Valorization of Larix decidua Mill. bark by functionalizing bioextract onto chitosan films for sustainable active food packaging. Carbohydr. Polym. 2021, 271, 118409. [Google Scholar] [CrossRef]
- Jakubowska, E.; Gierszewska, M.; Nowaczyk, J.; Olewnik-Kruszkowska, E. Physicochemical and storage properties of chitosan-based films plasticized with deep eutectic solvent. Food Hydrocoll. 2020, 108, 106007. [Google Scholar] [CrossRef]
- Lagaron, J.M.; Fernandez-Saiz, P.; Ocio, M.J. Using ATR-FTIR spectroscopy to design active antimicrobial food packaging structures based on high molecular weight chitosan polysaccharide. J. Agric. Food Chem. 2007, 55, 2554–2562. [Google Scholar] [CrossRef]
- Zarandona, I.; Minh, N.C.; Trung, T.S.; de la Caba, K.; Guerrero, P. Evaluation of bioactive release kinetics from crosslinked chitosan films with Aloe vera. Int. J. Biol. Macromol. 2021, 182, 1331–1338. [Google Scholar] [CrossRef]
- Costa, S.M.; Ferreira, D.P.; Teixeira, P.; Ballesteros, L.F.; Teixeira, J.A.; Fangueiro, R. Active natural-based films for food packaging applications: The combined effect of chitosan and nanocellulose. Int. J. Biol. Macromol. 2021, 177, 241–251. [Google Scholar] [CrossRef]
- You, G.; Feng, T.; Zhang, G.; Chen, M.; Liu, F.; Sun, L.; Wang, M.; Ren, X. Preparation, optimization, characterization and in vitro release of baicalein-solubilizing glycyrrhizic acid nano-micelles. Int. J. Pharm. 2021, 601, 120546. [Google Scholar] [CrossRef]
- Rhim, J.W.; Hong, S.I.; Park, H.M.; Ng, P.K.W. Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 5814–5822. [Google Scholar] [CrossRef]
- Mohamed, N.; Madian, N.G. Enhancement of the dynamic mechanical properties of chitosan thin films by crosslinking with greenly synthesized silver nanoparticles. J. Mater. Res. Technol. 2020, 9, 12970–12975. [Google Scholar] [CrossRef]
- Tan, W.; Dong, F.; Zhang, J.; Zhao, X.; Li, Q.; Guo, Z. Physical and antioxidant properties of edible chitosan ascorbate films. J. Agric. Food Chem. 2019, 67, 2530–2539. [Google Scholar] [CrossRef]
- Huo, C.; Yang, H. Synthesis and characterization of ZnO/palygorskite. Appl. Clay Sci. 2010, 50, 362–366. [Google Scholar] [CrossRef]
- Rosendo, F.R.; Pinto, L.I.; de Lima, I.S.; Trigueiro, P.; Honório, L.M.D.C.; Fonseca, M.G.; Silva-Filho, E.C.; Ribeiro, A.B.; Furtini, M.B.; Osajima, J.A. Antimicrobial efficacy of building material based on ZnO/palygorskite against Gram-negative and Gram-positive bacteria. Appl. Clay Sci. 2020, 188, 105499. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; El-Sayed, H.S.; Ibrahim, O.A.; Youssef, A.M. Rational design of chitosan/guar gum/zinc oxide bionanocomposites based on Roselle calyx extract for Ras cheese coating. Carbohydr. Polym. 2020, 239, 116234. [Google Scholar] [CrossRef]
- Lin, D.; Yang, Y.; Wang, J.; Yan, W.; Wu, Z.; Chen, H.; Zhang, Q.; Wu, D.; Qin, W.; Tu, Z. Preparation and characterization of TiO2-Ag loaded fish gelatin-chitosan antibacterial composite film for food packaging. Int. J. Biol. Macromol. 2020, 154, 123–133. [Google Scholar] [CrossRef]
- Taheri, P.; Jahanmardi, R.; Koosha, M.; Abdi, S. Physical, mechanical and wound healing properties of chitosan/gelatin blend films containing tannic acid and/or bacterial nanocellulose. Int. J. Biol. Macromol. 2020, 154, 421–432. [Google Scholar] [CrossRef]
- Mohamed, N.; Madian, N.G. Evaluation of the mechanical, physical and antimicrobial properties of chitosan thin films doped with greenly synthesized silver nanoparticles. Mater. Today Commun. 2020, 25, 101372. [Google Scholar] [CrossRef]
- Rodrigues, C.; Mello, J.M.M.; Dalcanton, F.; Lusitâneo, D.; Macuvele, D.L.P.; Padoin, N.; Fiori, M.A.; Soares, C.; Riella, H.G. Mechanical, thermal and antimicrobial properties of chitosan-based-nanocomposite with potential applications for food packaging. J. Polym. Environ. 2020, 28, 1216–1236. [Google Scholar] [CrossRef]
- Chang, X.; Hou, Y.; Liu, Q.; Hu, Z.; Xie, Q.; Shan, Y.; Li, G.; Ding, S. Physicochemical and antimicrobial properties of chitosan composite films incorporated with glycerol monolaurate and nano-TiO2. Food Hydrocolloids 2021, 119, 106846. [Google Scholar] [CrossRef]
- Kurek, M.; Brachais, C.H.; Nguimjeu, C.M.; Bonnotte, A.; Voilley, A.; Galić, K.; Couvercelle, J.P.; Debeaufort, F. Structure and thermal properties of a chitosan coated polyethylene bilayer film. Polym. Degrad. Stab. 2012, 97, 1232–1240. [Google Scholar] [CrossRef]
- Kavoosi, G.; Nateghpoor, B.; Dadfar, S.M.M.; Dadfar, S.M.A. Antioxidant, antifungal, water binding, and mechanical properties of poly (vinyl alcohol) film incorporated with essential oil as a potential wound dressing material. J. Appl. Polym. Sci. 2014, 131, 40937. [Google Scholar] [CrossRef]
- Abdurrahim, I. Water sorption, antimicrobial activity, and thermal and mechanical properties of chitosan/clay/glycerol nanocomposite films. Heliyon 2019, 5, e02342. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Wang, W.; Xu, J.; Wang, A. Mechanical and water resistance properties of chitosan/poly (vinyl alcohol) films reinforced with attapulgite dispersed by high-pressure homogenization. Chem. Eng. J. 2012, 210, 166–172. [Google Scholar] [CrossRef]
- Li, L.H.; Deng, J.C.; Deng, H.R.; Liu, Z.L.; Xin, L. Synthesis and characterization of chitosan/ZnO nanoparticle composite membranes. Carbohydr. Res. 2010, 345, 994–998. [Google Scholar] [CrossRef]
- Korting, H.C.; Schöllmann, C.; White, R.J. Management of minor acute cutaneous wounds: Importance of wound healing in a moist environment. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 130–137. [Google Scholar] [CrossRef]
- Tabatabaei, R.H.; Jafari, S.M.; Mirzaei, H.; Nafchi, A.M.; Dehnad, D. Preparation and characterization of nano-SiO2 reinforced gelatin-k-carrageenan biocomposites. Int. J. Biol. Macromol. 2018, 111, 1091–1099. [Google Scholar] [CrossRef]
- Faraji, S.; Nowroozi, N.; Nouralishahi, A.; Shayeh, J.S. Electrospun poly-caprolactone/graphene oxide/quercetin nanofibrous scaffold for wound dressing: Evaluation of biological and structural properties. Life Sci. 2020, 257, 118062. [Google Scholar] [CrossRef]
- Gasti, T.; Hiremani, V.D.; Sataraddi, S.P.; Vanjeri, V.N.; Goudar, N.; Masti, S.P.; Chougale, R.B.; Malabadi, R.B. UV screening, swelling and in-vitro cytotoxicity study of novel chitosan/poly (1-vinylpyrrolidone-co-vinyl acetate) blend films. Chem. Data Collect. 2021, 33, 100684. [Google Scholar] [CrossRef]
- Uzun, M.; Anand, S.C.; Shah, T. In vitro characterisation and evaluation of different types of wound dressing materials. J. Biomed. Eng. Technol. 2013, 1, 1–7. [Google Scholar] [CrossRef]
- Castano, O.; Pérez-Amodio, S.; Navarro-Requena, C.; Mateos-Timoneda, M.Á.; Engel, E. Instructive microenvironments in skin wound healing: Biomaterials as signal releasing platforms. Adv. Drug Deliv. Rev. 2018, 129, 95–117. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound dressings and comparative effectiveness data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [Green Version]
- Sahraee, S.; Ghanbarzadeh, B.; Milani, J.M.; Hamishehkar, H. Development of gelatin bionanocomposite films containing chitin and ZnO nanoparticles. Food Bioprocess Technol. 2017, 10, 1441–1453. [Google Scholar] [CrossRef]
- Jing, Y.; Mu, B.; Zhang, M.; Wang, L.; Zhong, H.; Liu, X.; Wang, A. Zinc-loaded palygorskite nanocomposites for catheter coating with excellent antibacterial and anti-biofilm properties. Colloid Surf. A Physicochem. Eng. Asp. 2020, 600, 124965. [Google Scholar] [CrossRef]
- Qing, X.; He, G.; Liu, Z.; Yin, Y.; Cai, W.; Fan, L.; Fardim, P. Preparation and properties of polyvinyl alcohol/N–succinyl chitosan/lincomycin composite antibacterial hydrogels for wound dressing. Carbohydr. Polym. 2021, 261, 117875. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Truşcă, R.D.; Ilie, C.I.; Oprea, O.C.; Andronescu, E. Innovative antimicrobial chitosan/ZnO/Ag NPs/Citronella essential oil nanocomposite—Potential coating for grapes. Foods 2020, 9, 1801. [Google Scholar] [CrossRef]
- Vasile, B.S.; Oprea, O.; Voicu, G.; Ficai, A.; Andronescu, E.; Teodorescu, A.; Holban, A. Synthesis and characterization of a novel controlled release zinc oxide/gentamicin–chitosan composite with potential applications in wounds care. Int. J. Pharm. 2014, 463, 161–169. [Google Scholar] [CrossRef]
- Yilmaz, A.H. Antibacterial activity of chitosan-based systems. Funct. Chitosan 2020, 15, 457–489. [Google Scholar] [CrossRef]
- Radulescu, M.; Ficai, D.; Oprea, O.; Ficai, A.; Andronescu, E.; Holban, A.M. Antimicrobial chitosan based formulations with impact on different biomedical applications. Curr. Pharm. Biotechnol. 2015, 16, 128–136. [Google Scholar] [CrossRef]
- Kim, H.K.; Park, Y.; Kim, H.N.; Choi, B.H.; Jeong, H.G.; Lee, D.G.; Hahm, K.S. Antimicrobial mechanism of β-glycyrrhetinic acid isolated from licorice, Glycyrrhiza glabra. Biotechnol. Lett. 2002, 24, 1899–1902. [Google Scholar] [CrossRef]
- Jiang, S.; Lin, K.; Cai, M. ZnO nanomaterials: Current advancements in antibacterial mechanisms and applications. Front. Chem. 2020, 8, 580. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, G.; Wei, Z.; Sang, L.; Qi, M. Hemocompatibility evaluation of polyurethane film with surface-grafted poly (ethylene glycol) and carboxymethyl-chitosan. J. Appl. Polym. Sci. 2013, 127, 308–315. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-contacting biomaterials: In vitro evaluation of the hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]







| Samples | WC (%) | WS (%) | SD (%) |
|---|---|---|---|
| CG | 11.21 ± 0.91 e | 28.15 ± 0.92 a | 325.94 ± 1.77 f |
| CGZP−0.25% | 12.92 ± 0.89 d | 27.35 ± 1.58 a | 384.78 ± 2.07 e |
| CGZP−0.5% | 13.78 ± 0.27 c,d | 26.67 ± 0.92 a,b | 394.57 ± 3.90 d |
| CGZP−1.0% | 14.87 ± 0.66 b,c | 24.39 ± 0.90 b,c | 415.39 ± 3.79 c |
| CGZP−2.5% | 15.75 ± 0.53 a,b | 22.12 ± 0.41 c,d | 433.54 ± 4.43 b |
| CGZP−5.0% | 17.13 ± 0.39 a | 21.58 ± 1.19 d | 472.49 ± 3.32 a |
| Samples | Transmittance (%) | Thickness (mm) | Opacity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 200 nm | 300 nm | 400 nm | 500 nm | 600 nm | 700 nm | 800 nm | |||
| CG | 0.02 | 58.05 | 78.01 | 88.76 | 89.94 | 90.31 | 90.54 | 0.0548 ± 0.0018 a | 0.84 ± 0.03 e |
| CGZP−0.25% | 0.02 | 58.20 | 77.67 | 87.93 | 88.96 | 89.34 | 89.51 | 0.0537 ± 0.0016 a,b | 0.95 ± 0.03 d |
| CGZP−0.5% | 0.02 | 58.93 | 77.99 | 87.55 | 88.68 | 89.08 | 89.38 | 0.0533 ± 0.0018 a,b | 0.98 ± 0.03 c,d |
| CGZP−1.0% | 0.01 | 58.98 | 78.12 | 87.84 | 88.93 | 89.38 | 89.59 | 0.0529 ± 0.0017 b | 0.96 ± 0.03 c |
| CGZP−2.5% | 0.02 | 54.26 | 76.02 | 86.80 | 88.07 | 88.54 | 88.91 | 0.0527 ± 0.0013 b | 1.05 ± 0.03 b |
| CGZP−5.0% | 0.02 | 54.46 | 74.69 | 84.00 | 85.26 | 85.74 | 86.08 | 0.0533 ± 0.0018 a,b | 1.30 ± 0.04 a |
| Inhibitory Zones in Diameter (mm) | E. coli | S. aureus | ESBL—E. coli | MRSA |
|---|---|---|---|---|
| CG | 13.44 ± 0.53 b,c | 13.70 ± 0.32 b | 12.54 ± 0.34 b,c | 12.36 ± 0.31 c |
| CGZP−0.25% | 12.64 ± 0.86 c | 13.13 ± 0.45 b | 11.72 ± 0.64 c | 12.06 ± 0.55 c |
| CGZP−0.5% | 13.36 ± 0.31 b,c | 13.55 ± 0.38 b | 12.08 ± 0.52 b,c | 12.30 ± 0.44 c |
| CGZP−1.0% | 13.50 ± 0.32 b,c | 13.73 ± 0.15 b | 12.30 ± 0.44 a,b | 12.62 ± 0.53 b,c |
| CGZP−2.5% | 14.14 ± 0.51 a,b | 14.68 ± 0.38 a | 12.84 ± 0.72 a,b | 13.12 ± 0.57 a,b |
| CGZP−5.0% | 15.04 ± 1.07 a | 14.50 ± 0.42 a | 13.28 ± 0.31 a | 13.64 ± 0.29 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Zhang, H.; Hui, A.; Ding, J.; Liu, X.; Wang, A. Synergistic Effect of Glycyrrhizic Acid and ZnO/Palygorskite on Improving Chitosan-Based Films and Their Potential Application in Wound Healing. Polymers 2021, 13, 3878. https://doi.org/10.3390/polym13223878
Zhang Q, Zhang H, Hui A, Ding J, Liu X, Wang A. Synergistic Effect of Glycyrrhizic Acid and ZnO/Palygorskite on Improving Chitosan-Based Films and Their Potential Application in Wound Healing. Polymers. 2021; 13(22):3878. https://doi.org/10.3390/polym13223878
Chicago/Turabian StyleZhang, Qian, Hong Zhang, Aiping Hui, Junjie Ding, Xinyue Liu, and Aiqin Wang. 2021. "Synergistic Effect of Glycyrrhizic Acid and ZnO/Palygorskite on Improving Chitosan-Based Films and Their Potential Application in Wound Healing" Polymers 13, no. 22: 3878. https://doi.org/10.3390/polym13223878