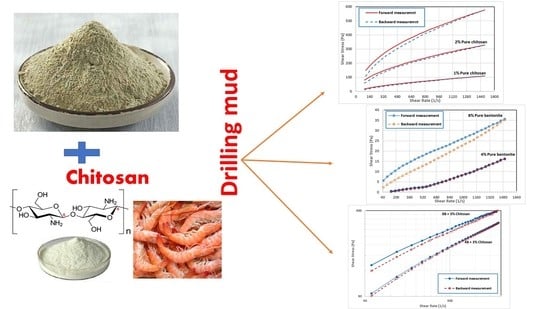
The Effect of Biopolymer Chitosan on the Rheology and Stability of Na-Bentonite Drilling Mud
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Suspension Preparation
2.3. Rheological Measurements
3. Results and Discussion
3.1. Rheology of Pure Chitosan
3.2. Rheology of Pure Bentonite
3.3. Rheology of Bentonite-Chitosan Dispersions
3.4. Stability of Bentonite-Chitosan Dispersions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reddy, D.H.K.; Lee, S.M.; Kim, J.O. A review on emerging applications of natural sericite and its composites. World Appl. Sci. J. 2013, 27, 1514–1523. [Google Scholar]
- Hassan, M.S.; Abdel-Khalek, N.A. Beneficiation and applications of an Egyptian bentonite. Appl. Clay Sci. 1998, 13, 99–115. [Google Scholar] [CrossRef]
- Menezes, R.R.; Marques, L.N.; Campos, L.A.; Ferreira, H.S.; Santana, L.N.L.; Neves, G.A. Use of statistical design to study the influence of CMC on the rheological properties of bentonite dispersions for water-based drilling fluids. Appl. Clay Sci. 2010, 49, 13–20. [Google Scholar] [CrossRef]
- Cara, S.; Carcangiu, G.; Padalino, G.; Palomba, M.; Tamanini, M. The bentonites in pelotherapy: Chemical, mineralogical and technological properties of materials from Sardinia deposits (Italy). Appl. Clay Sci. 2000, 16, 117–124. [Google Scholar] [CrossRef]
- Kaci, A.; Chaouche, M.; Andréani, P.A. Influence of bentonite clay on the rheological behaviour of fresh mortars. Cem. Concr. Res. 2011, 41, 373–379. [Google Scholar] [CrossRef]
- Darvishi, Z.; Morsali, A. Synthesis and characterization of Nano-bentonite by sonochemical method. Ultrason. Sonochem. 2011, 18, 238–242. [Google Scholar] [CrossRef]
- Abu-Jdayil, B.; Al-Malah, K.; Sawalha, R. Study on bentonite-unsaturated polyester composite materials. J. Reinf. Plast. Compos. 2002, 21, 1597–1607. [Google Scholar] [CrossRef]
- Al-Malah, K.; Abu-Jdayil, B. Clay-based heat insulator composites: Thermal and water retention properties. Appl. Clay Sci. 2007, 37, 90–96. [Google Scholar] [CrossRef]
- Soy, U.; Demir, A.; Caliskan, F. Effect of bentonite addition on fabrication of reticulated porous SiC ceramics for liquid metal infiltration. Ceram. Int. 2011, 37, 15–19. [Google Scholar] [CrossRef]
- Banat, F.; Al-Asheh, S.; Abu-Aitah, L. Competitive adsorption of phenol, copper ions and nickel ions on to heat-treated bentonite. Adsorpt. Sci. Technol. 2002, 20, 107–117. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Hage, A.P.; Zhou, M.; Sonawane, S.H.; Ashokkumar, M. Ultrasound assisted preparation of nanoclay Bentonite-FeCo nanocomposite hybrid hydrogel: A potential responsive sorbent for removal of organic pollutant from water. Desalination 2011, 281, 429–437. [Google Scholar] [CrossRef]
- Guo, J.; Chen, S.; Liu, L.; Li, B.; Yang, P.; Zhang, L.; Feng, Y. Adsorption of dye from wastewater using chitosan–CTAB modified bentonites. J. Colloid Interface Sci. 2012, 382, 61–66. [Google Scholar] [CrossRef]
- Luckham, P.F.; Rossi, S. The colloidal and rheological properties of bentonite suspensions. Adv. Colloid Interface Sci. 1999, 82, 43–92. [Google Scholar] [CrossRef] [Green Version]
- Coussot, P.; Bertrand, F.; Herzhaft, B. Rheological behavior of drilling muds, characterization using MRI visualization. Oil Gas Sci. Technol. 2004, 59, 23–29. [Google Scholar] [CrossRef]
- Mohammed, A.S. Electrical resistivity and rheological properties of sensing bentonite drilling muds modified with lightweight polymer. Egypt. J. Pet. 2018, 27, 55–63. [Google Scholar] [CrossRef]
- Abu-Jdayil, B.; Ghannam, M. Effect of Surfactants on the Performance of Water-Based Drilling Fluids. In Surfactants in Upstream E&P; Springer International Publishing: Cham, Switzerland, 2021; pp. 73–111. [Google Scholar]
- Abu-Jdayil, B. Rheology of sodium and calcium bentonite–water dispersions: Effect of electrolytes and aging time. Int. J. Miner. Process. 2011, 98, 208–213. [Google Scholar] [CrossRef]
- Abdou, M.I.; El-Sayed Ahmed, H. Effect of particle size of bentonite on rheological behavior of the drilling mud. Pet. Sci. Technol. 2011, 29, 2220–2233. [Google Scholar] [CrossRef]
- Choo, K.Y.; Bai, K. Effects of bentonite concentration and solution pH on the rheological properties and long-term stabilities of bentonite suspensions. Appl. Clay Sci. 2015, 108, 182–190. [Google Scholar] [CrossRef]
- Maxey, J. Thixotropy and yield stress behavior in drilling fluids. In Proceedings of the AADE 2007 Drilling Fluids Conference (AADE-07-NTCE-37), Houston, TX, USA, 10–12 April 2007. [Google Scholar]
- Abdou, M.I.; Ahmed, H.E.S. A study on the thixotropy of Egyptian bentonite suspensions. Pet. Sci. Technol. 2013, 31, 1980–1991. [Google Scholar] [CrossRef]
- Hammadi, L.; Boudjenane, N.; Houdjedje, R.; Reffis, R.; Belhadri, M. Modeling the Time-Dependent Rheological Behavior of Clays Used in Fabrication of Ceramic. Int. J. Mech. Aerosp. Industrial. Mechatron. Manuf. Eng. 2015, 9, 1373–1376. [Google Scholar]
- Haveman, J.E. Effect of drilling fluid contamination by zinc ions on drilling fluid rheological behaviour. Master’s Thesis, Department of Water Management, Delft University of Technology, Delft, The Netherlands, August 2016. Available online: http://repository.tudelft.nl/ (accessed on 25 May 2021).
- Güngör, N.; Alemdar, A. Influences of electrolytes, polymers and a surfactant on rheological properties of bentonite–water systems. J. Incl. Phenom. Macrocycl. Chem. 1999, 33, 155–168. [Google Scholar]
- İşci, S.; Günister, E.; Ece, Ö.I.; Güngör, N. The modification of rheological properties of clays with PVA effect. Mater. Lett. 2004, 58, 1975–1978. [Google Scholar] [CrossRef]
- Saito, M.; Asai, M. Drilling Fluid Additive. U.S. Patent No. 9,475,900, 25 October 2016. [Google Scholar]
- M’bodj, O.; Ariguib, N.K.; Ayadi, M.T.; Magnin, A. Plastic and elastic properties of the systems interstratified clay–water–electrolyte–xanthan. J. Colloid Interface Sci. 2004, 273, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Mahto, V.; Sharma, V.P. Rheological study of a water based oil well drilling fluid. J. Pet. Sci. Eng. 2004, 45, 123–128. [Google Scholar] [CrossRef]
- Alemdar, A.; Öztekin, N.; Güngör, N.; Ece, Ö.I.; Erim, F.B. Effects of polyethyleneimine adsorption on the rheological properties of purified bentonite suspensions. Colloids Surf. A Physicochem. Eng. Asp. 2005, 252, 95–98. [Google Scholar] [CrossRef]
- Tunc, S.; Duman, O. The effect of different molecular weight of poly (ethylene glycol) on the electrokinetic and rheological properties of Na-bentonite suspensions. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 93–99. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, S.; Chen, Y.; Han, D.; Wang, D. Impacts of polypropylene glycol (PPG) additive and pH on tribological properties of water-based drilling mud for steel-steel contact. Tribol. Int. 2017, 110, 318–325. [Google Scholar] [CrossRef]
- Benyounes, K.; Mellak, A.; Benchabane, A. The effect of carboxymethylcellulose and xanthan on the rheology of bentonite suspensions. Energy Sources Part A Recovery Util. Environ. Eff. 2010, 32, 1634–1643. [Google Scholar] [CrossRef]
- Abu-Jdayil, B.; Ghannam, M. The modification of rheological properties of sodium bentonite-water dispersions with low viscosity CMC polymer effect. Energy Sources Part A Recovery Util. Environ. Eff. 2014, 36, 1037–1048. [Google Scholar] [CrossRef]
- Shaikh, S.M.; Nasser, M.S.; Hussein, I.A.; Benamor, A. Investigation of the effect of polyelectrolyte structure and type on the electrokinetics and flocculation behavior of bentonite dispersions. Chem. Eng. J. 2017, 311, 265–276. [Google Scholar] [CrossRef]
- Xie, G.; Luo, P.; Deng, M.; Su, J.; Wang, Z.; Gong, R.; Duan, Q. Intercalation behavior of branched polyethyleneimine into sodium bentonite and its effect on rheological properties. Appl. Clay Sci. 2017, 141, 95–103. [Google Scholar] [CrossRef]
- Günister, E.; Alemdar, S.A.; Güngör, N. Effect of sodium dodecyl sulfate on flow and electrokinetic properties of Na-activated bentonite dispersions. Bull. Mater. Sci. 2004, 27, 317–322. [Google Scholar] [CrossRef]
- Abu-Jdayil, B.; Ghannam, M.; Nasser, M.S. The Modification of Rheological Properties of Bentonite-Water Dispersions with Cationic and Anionic Surfactants. Int. J. Chem. Eng. Appl. 2016, 7, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Glass, J.E., Jr. HEC-Bentonite Compatible Blends. U.S. Patent No. 4,561,985, 31 December 1985. [Google Scholar]
- Hossain, M.E.; Wajheeuddin, M. The use of grass as an environmentally friendly additive in water-based drilling fluids. Pet. Sci. 2016, 13, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Ghassemzadeh, J. Lost Circulation Material for Oilfield Use. U.S. Patent 7,923,413 B2, Assigned to Schlumberger Technology Corporation, 12 April 2011. [Google Scholar]
- Gu, X.; Cheng, C.; Zhang, J.; Zhang, J.; Chen, G.; Ma, C.; Zhang, Z. Stabilization of Montmorillonite by Ammoniated Lignosulfonates and Its Use in Water-Based Drilling Fluid. Sci. Adv. Mater. 2017, 9, 928–933. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Dutta, P.K.; Ravikumar, M.N.V.; Dutta, J. Chitin and chitosan for versatile applications. J. Macromol. Sci. Part C Polym. Rev. 2002, 42, 307–354. [Google Scholar] [CrossRef]
- Uragami, T.; Kurita, K.; Fukamizo, T. Chitin and chitosan in life science. In Proceedings of the 8th International Chitin and Chitosan Conference, Kodansha Scientific Ltd, Tokyo, Japan, 1 January 2001. [Google Scholar]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Sansano, M.; Heredia, A.; Glicerina, V.; Balestra, F.; Romani, S.; Andrés, A. Influence of chitosan on thermal, microstructural and rheological properties of rice and wheat flours-based batters. LWT-Food Sci. Technol. 2018, 87, 529–536. [Google Scholar] [CrossRef]
- Tirtom, V.N.; Dinçer, A.; Becerik, S.; Aydemir, T.; Çelik, A. Comparative adsorption of Ni (II) and Cd (II) ions on epichlorohydrin crosslinked chitosan–clay composite beads in aqueous solution. Chem. Eng. J. 2012, 197, 379–386. [Google Scholar] [CrossRef]
- Bée, A.; Obeid, L.; Mbolantenaina, R.; Welschbillig, M.; Talbot, D. Magnetic chitosan/clay beads: A magsorbent for the removal of cationic dye from water. J. Magn. Magn. Mater. 2017, 421, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Kelessidis, V.C.; Papanicolaou, C.; Foscolos, A. Application of Greek lignite as an additive for controlling rheological and filtration properties of water–bentonite suspensions at high temperatures: A review. Int. J. Coal Geol. 2009, 77, 394–400. [Google Scholar] [CrossRef]
- Torres, M.A.; Beppu, M.M.; Arruda, E.J. Viscous and viscoelastic properties of chitosan solutions and gels. Braz. J. Food Technol. 2006, 9, 101–108. [Google Scholar]
- Elhafian, E.A.; Elgannoudi, E.S.; Mainal, A.; Yahaya, A.H.B. Characterization of chitosan in acetic acid: Rheological and thermal studies. Turk. J. Chem. 2010, 34, 47–56. [Google Scholar]
- Abu-Jdayil, B.; Fara, D.A. Modification of the rheological behaviour of sodium alginate by chitosan and multivalent electrolytes. Ital. J. Food Sci. 2013, 25, 196–201. [Google Scholar]
- Wang, B.; Qiao, C.; Gao, X.; Yang, X.; Li, Y.; Li, T. Rheological properties of N-[(2-hydroxyl)-propyl-3-trimethyl ammonium] chitosan chloride. Carbohydr. Polym. 2017, 171, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Delben, F.; Lapasin, R.; Pricl, S. Flow properties of N-(carboxymethyl) chitosan aqueous systems in the sol and gel domains. Int. J. Biol. Macromol. 1990, 12, 9–13. [Google Scholar] [CrossRef]
- Kjøniksen, A.L.; Nyström, B.; Iversen, C.; Nakken, T.; Palmgren, O.; Tande, T. Viscosity of dilute aqueous solutions of hydrophobically modified chitosan and its unmodified analogue at different conditions of salt and surfactant concentrations. Langmuir 1997, 13, 4948–4952. [Google Scholar] [CrossRef]
- Mucha, M. Rheological characteristics of semi-dilute chitosan solutions. Macromol. Chem. Phys. 1997, 198, 471–484. [Google Scholar] [CrossRef]
- Afzal, S.; Maswal, M.; Dar, A.A. Rheological behavior of pH responsive composite hydrogels of chitosan and alginate: Characterization and its use in encapsulation of citral. Colloids Surf. B Biointerfaces 2018, 169, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, X.; Qiao, C.; Li, Y.; Li, T.; Xu, C. Effects of chitosan quaternary ammonium salt on the physicochemical properties of sodium carboxymethyl cellulose-based films. Carbohydr. Polym. 2018, 184, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Taherian, A.R.; Lacasse, P.; Bisakowski, B.; Pelletier, M.; Lanctôt, S.; Fustier, P. Rheological and thermogelling properties of commercials chitosan/β-glycerophosphate: Retention of hydrogel in water, milk and UF-milk. Food Hydrocoll. 2017, 63, 635–645. [Google Scholar] [CrossRef]
- Bezerril, L.M.; De Vasconcelos, C.L.; Dantas, T.N.C.; Pereira, M.R.; Fonseca, J.L.C. Rheology of chitosan-kaolin dispersions. Colloids Surf. A Physicochem. Eng. Asp. 2006, 287, 24–28. [Google Scholar] [CrossRef]
- Azzam, E.M.; Solyman, S.M.; Abd-Elaal, A.A. Fabrication of chitosan/Ag-nanoparticles/clay nanocomposites for catalytic control on oxidative polymerization of aniline. Colloids Surf. A Physicochem. Eng. Asp. 2016, 510, 221–230. [Google Scholar] [CrossRef]
- API. Specification for Drilling Fluids—Specifications and Testing. In API Specification 13A, 18th ed.; API: Washington, DC, USA, 2010. [Google Scholar]
- Tiwari, A.P.; Ghosh, S.J.; Pawar, S.H. Synthesis and characterization of functionalized Superparamagnetic nanoparticles for isolation of DNA. Int. J. Pharm. Bio. Sci. 2014, 5, 533–542. [Google Scholar]
- Tunç, S.; Duman, O.; Kancı, B. Rheological measurements of Na-bentonite and sepiolite particles in the presence of tetradecyltrimethylammonium bromide, sodium tetradecyl sulfonate and Brij 30 surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2012, 398, 37–47. [Google Scholar] [CrossRef]











| Composition | H2O | Trace Elements | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| wt% | 2.425 | 0.245 | 3.250 | 2.670 | 21.080 | 63.020 | 0.650 | 0.350 | 5.64 | 0.720 |
| Chitosan Conc. (wt.%) | Range (s−1) | m (Pa·sn) | n | A (Hysteresis Loop Area) (Pa/s) |
|---|---|---|---|---|
| 0.10 | 700–1550 | 0.0104 | 1.0 | - |
| 0.20 | 700–1550 | 0.0143 | 1.0 | - |
| 0.50 | 700–1550 | 0.1075 | 0.83 | - |
| 1.00 | 100–1550 | 0.8792 | 66 | 3202 |
| 2.00 | 100–1550 | 7.1727 | 0.52 | 11,330 |
| 3.00 | 57.2–1550 | 12.0380 | 0.53 | 24,240 |
| Solid Conc. (wt.%) | Chitosan Conc. (wt.%) | Range (s−1) | (Pa) | m (Pa·sn) | n | A (Hysteresis Loop Area) (Pa/s) |
|---|---|---|---|---|---|---|
| 4.0 | 0.0 | 47.8–560 | 1.60 | 0.0033 | 1.0 | 577 |
| 0.0 | 560–1500 | 0.0 | 0.0001 | 1.7 | ||
| 0.1 | 329–1500 | 0.0 | 2.0446 | 165 | ||
| 0.2 | 142–1500 | 0.0 | 1.8085 | 179 | ||
| 0.5 | 47.8–1500 | 0.0 | 0.0154 | 1.0348 | 252 | |
| 1.0 | 47.8–1500 | 0.0 | 0.2011 | 0.8344 | 912 | |
| 2.0 | 47.8–1500 | 0.0 | 0.6979 | 0.7824 | 1600 | |
| 3.0 | 47.8–1500 | 16.50 | 21.7080 | 0.4608 | 12.090 | |
| 8.0 | 0.0 | 47.8–1500 | 8.65 | 0.0652 | 0.82 | 3189 |
| 0.1 | 142–1500 | 0.0 | 0.0003 | 1.533 | 3005 | |
| 0.2 | 94.7–1500 | 0.0 | 0.0075 | 1.058 | 1255 | |
| 0.5 | 94.7–1500 | 0.0 | 0.0164 | 1.038 | 1178 | |
| 1.0 | 47.8–1500 | 0.0 | 0.1842 | 0.8676 | 4853 | |
| 2.0 | 47.8–1500 | 15.2 | 5.1782 | 0.5552 | 6223 | |
| 3.0 | 47.8–1500 | 25.50 | 64.772 | 0.3583 | 44,140 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Jdayil, B.; Ghannam, M.; Alsayyed Ahmed, K.; Djama, M. The Effect of Biopolymer Chitosan on the Rheology and Stability of Na-Bentonite Drilling Mud. Polymers 2021, 13, 3361. https://doi.org/10.3390/polym13193361
Abu-Jdayil B, Ghannam M, Alsayyed Ahmed K, Djama M. The Effect of Biopolymer Chitosan on the Rheology and Stability of Na-Bentonite Drilling Mud. Polymers. 2021; 13(19):3361. https://doi.org/10.3390/polym13193361
Chicago/Turabian StyleAbu-Jdayil, Basim, Mamdouh Ghannam, Karam Alsayyed Ahmed, and Mohamed Djama. 2021. "The Effect of Biopolymer Chitosan on the Rheology and Stability of Na-Bentonite Drilling Mud" Polymers 13, no. 19: 3361. https://doi.org/10.3390/polym13193361