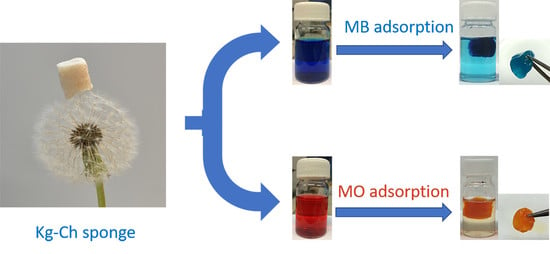
Eco-Friendly and Economic, Adsorptive Removal of Cationic and Anionic Dyes by Bio-Based Karaya Gum—Chitosan Sponge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Deacetylated Karaya Gum
2.3. Preparation of Chitosan Solution
2.4. Synthesis of Kg-Ch Sponge
2.5. Zeta Potential
2.6. Determination of Density
2.7. Determination of Porosity
2.8. Swelling Measurements
2.9. Stability Studies
2.10. ATR-FTIR (Attenuated Total Reflection-Fourier Transform Infrared) Spectroscopy
2.11. FE-SEM (Scanning Electron Microscopy)
2.12. Thermogravimetric Analysis (TGA)
2.13. Adsorption Studies of Dye Solutions
2.14. Adsorption Isotherm
2.15. Kinetic Studies
2.16. Regeneration and Reusability
3. Results and Discussion
3.1. Kg-Ch Sponge
3.2. Density and Porosity
3.3. ATR-FTIR Analysis
3.4. Chemical Stability
3.5. SEM Analysis
3.6. Thermogravimetric Analysis
3.7. Swelling Studies
3.8. Adsorption Properties of Kg-Ch Sponges
3.8.1. Effect of pH
3.8.2. Effect of Adsorbent Dosage
3.8.3. Effect of the Initial Concentration of Adsorbates
3.9. Adsorption Isotherm Evaluation
3.10. Adsorption Kinetics
3.11. The Mechanism for the Adsorption of Dyes onto the Kg-Ch Sponge
3.12. Effect of Adsorption-Desorption Cycles on the Dye Removal Efficiency of Kg-Ch Sponge
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghorai, S.; Sarkar, A.; Raou, M.; Panda, A.B.; Scho, H.; Pal, S. Enhanced Removal of Methylene Blue and Methyl Violet Dyes from Aqueous Solution Using a Nanocomposite of Hydrolyzed Polyacrylamide Grafted Xanthan Gum and Incorporated Nanosilica. ACS Appl. Mater. Interfaces 2014, 6, 4766–4777. [Google Scholar] [CrossRef]
- Blackburn, R.S. Natural polysaccharides and their interactions with dye molecules: Applications in effluent treatment. Environ. Sci. Technol. 2004, 38, 4905–4909. [Google Scholar] [CrossRef]
- Vakili, M.; Zwain, H.M.; Mojiri, A.; Wang, W.; Gholami, F.; Gholami, Z.; Giwa, A.S.; Wang, B.; Cagnetta, G.; Salamatinia, B. Effective Adsorption of Reactive Black 5 onto Hybrid Hexadecylamine Impregnated Chitosan-Powdered Activated Carbon Beads. Water 2020, 12, 2242. [Google Scholar] [CrossRef]
- Sekar, S.; Surianarayanan, M.; Ranganathan, V.; Macfarlane, D.R.; Mandal, A.B. Choline-Based Ionic Liquids-Enhanced Biodegradation of Azo Dyes. Environ. Sci. Technol. 2012, 46, 4902–4908. [Google Scholar] [CrossRef] [PubMed]
- Körbahti, B.K.; Artut, K.; Gec, C.; Özer, A. Electrochemical decolorization of textile dyes and removal of metal ions from textile dye and metal ion binary mixtures. Chem. Eng. J. 2011, 173, 677–688. [Google Scholar] [CrossRef]
- Caldera Villalobos, M.; Peláez Cid, A.A.; Herrera González, A.M. Removal of textile dyes and metallic ions using polyelectrolytes and macroelectrolytes containing sulfonic acid groups. J. Environ. Manag. 2016, 177, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Polepalli, S.; Rao, C.P. Drum Stick Seed Powder as Smart Material for Water Purification: Role of Moringa oleifera Coagulant Protein-Coated Copper Phosphate Nanoflowers for the Removal of Heavy Toxic Metal Ions and Oxidative Degradation of Dyes from Water. ACS Sustain. Chem. Eng. 2018, 6, 15634–15643. [Google Scholar] [CrossRef]
- Tang, C.-W. Study of degradation of photocatalytic methyl orange on Different Morphologies of ZnO Catalysts. Adv. Mater. Res. 2013, 2, 19–24. [Google Scholar] [CrossRef]
- Blachnio, M.; Budnyak, T.M.; Derylo-Marczewska, A.; Marczewski, A.W.; Tertykh, V.A. Chitosan-Silica Hybrid Composites for Removal of Sulfonated Azo Dyes from Aqueous Solutions. Langmuir 2018, 34, 2258–2273. [Google Scholar] [CrossRef]
- Sharma, J.; Kaith, B.S.; Sharma, A.K. Gum xanthan-psyllium-cl-poly (acrylic acid-co-itaconic acid) based adsorbent for e ff ective removal of cationic and anionic dyes: Adsorption isotherms, kinetics and thermodynamic studies. Ecotoxicol. Environ. Saf. 2018, 149, 150–158. [Google Scholar]
- Shen, X.; Huang, P.; Li, F.; Wang, X.; Yuan, T.; Sun, R. Compressive alginate sponge derived from seaweed biomass resources for methylene blue removal from wastewater. Polymers 2019, 11, 961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaedi, M.; Nazari, E.; Sahraie, R.; Purkait, M.K. Kinetic and isotherm study of Bromothymol Blue and Methylene blue removal using Au-NP loaded on activated carbon. Desalin. Water Treat. 2014, 52, 5504–5512. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, H.; Pan, Y.; Wang, L. Novel Composite Adsorbent Consisting of Dissolved Cellulose Fiber/Microfibrillated Cellulose for Dye Removal from Aqueous Solution. ACS Sustain. Chem. Eng. 2018, 6, 6994–7002. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Dehabadi, L.; Wilson, L.D. Renewable Starch Carriers with Switchable Adsorption Properties. ACS Sustain. Chem. Eng. 2018, 6, 4603–4613. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, D.; Qiu, X. Hydroxypropyl Sulfonated Lignin as Dye Dispersant: Effect of Average Molecular Weight. ACS Sustain. Chem. Eng. 2015, 3, 3239–3244. [Google Scholar] [CrossRef]
- Ayoub, A.; Venditti, R.A.; Pawlak, J.J.; Salam, A.; Hubbe, M.A. Novel Hemicellulose—Chitosan Biosorbent for Water Desalination and Heavy Metal Removal. ACS Sustain. Chem. Eng. 2013, 1, 1102–1109. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Wang, S.; Peng, X.; Zhong, L.; Tan, J.; Jing, S. An ultralight, elastic, cost-effective, and highly recyclable superabsorbent from microfibrillated cellulose fibers for oil spillage cleanup. J. Mater. Chem. A 2015, 3, 8772–8781. [Google Scholar] [CrossRef]
- Salehizadeh, H.; Yan, N.; Farnood, R. Recent advances in polysaccharide bio-based flocculants. Biotechnol. Adv. 2018, 36, 92–119. [Google Scholar] [CrossRef]
- Padil, V.V.T.T.; Wacławek, S.; Černík, M.; Varma, R.S.; Miroslav, Č.; Varma, R.S.; Wacławek, S.; Černík, M.; Varma, R.S. Tree gum-based renewable materials: Sustainable applications in nanotechnology, biomedical and environmental fi elds. Biotechnol. Adv. 2018, 36, 1984–2016. [Google Scholar] [CrossRef]
- Wang, L.; Sánchez-soto, M.; Abt, T. Properties of bio-based gum Arabic/clay aerogels. Ind. Crops Prod. 2016, 91, 15–21. [Google Scholar] [CrossRef]
- Costa, M.P.M.; Prates, L.M.; Baptista, L.; Cruz, M.T.M.; Ferreira, I.L.M. Interaction of polyelectrolyte complex between sodium alginate and chitosan dimers with a single glyphosate molecule: A DFT and NBO study. Carbohydr. Polym. 2018, 198, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Das, B.P.; Tsianou, M. From polyelectrolyte complexes to polyelectrolyte multilayers: Electrostatic assembly, nanostructure, dynamics, and functional properties. Adv. Colloid Interface Sci. 2017, 244, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef]
- Kumar, A.; Ahuja, M. Carboxymethyl gum kondagogu-chitosan polyelectrolyte complex nanoparticles: Preparation and characterization. Int. J. Biol. Macromol. 2013, 62, 80–84. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, B.; Zhang, Y.; He, Y.; Huang, L.; Tan, S.; Cai, X. Simultaneous removal of cationic and anionic dyes from environmental water using montmorillonite-pillared graphene oxide. J. Chem. Eng. Data 2015, 60, 1270–1278. [Google Scholar] [CrossRef]
- Zhao, L.; Zou, W.; Zou, L.; He, X.; Song, J.; Han, R. Adsorption of methylene blue and methyl orange from aqueous solution by iron oxide-coated zeolite in fi xed bed column: Predicted curves. Desalin. Water Treat. 2010, 22, 258–264. [Google Scholar] [CrossRef]
- Bidarakatte Krishnappa, P.; Badalamoole, V. Karaya gum-graft-poly(2-(dimethylamino)ethyl methacrylate) gel: An efficient adsorbent for removal of ionic dyes from water. Int. J. Biol. Macromol. 2018, 122, 997–1007. [Google Scholar] [CrossRef]
- Mojiri, A.; Kazeroon, R.A.; Gholami, A. Cross-linked magnetic chitosan/activated biochar for removal of emerging micropollutants from water: Optimization by the artificial neural network. Water 2019, 11, 551. [Google Scholar] [CrossRef] [Green Version]
- Brar, V.; Kaur, G. Preparation and Characterization of Polyelectrolyte Complexes of Hibiscus esculentus (Okra) Gum and Chitosan. Int. J. Biomater. 2018, 2018, 4856287. [Google Scholar] [CrossRef] [Green Version]
- Padil, V.V.T.; Senan, C.; Černík, M. Dodecenylsuccinic Anhydride Derivatives of Gum Karaya (Sterculia urens): Preparation, Characterization, and Their Antibacterial Properties. J. Agric. Food Chem. 2015, 63, 3757–3765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Uch, B.; Agarwal, S.; Greiner, A. Ultralight, Thermally Insulating, Compressible Polyimide Fiber Assembled Sponges. ACS Appl. Mater. Interfaces 2017, 9, 32308–32315. [Google Scholar] [CrossRef]
- Pietrucha, K.; Safandowska, M. Dialdehyde cellulose-crosslinked collagen and its physicochemical properties. Process Biochem. 2015, 50, 2105–2111. [Google Scholar] [CrossRef]
- Sakloetsakun, D.; Preechagoon, D.; Bernkop-schnu, A.; Pongjanyakul, T. Chitosan-gum arabic polyelectrolyte complex films: Physicochemical, mechanical and mucoadhesive properties. Pharm. Dev. Technol. 2016, 21, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ahuja, M. Carboxymethyl gum kondagogu: Synthesis, characterization and evaluation as mucoadhesive polymer. Carbohydr. Polym. 2012, 90, 637–643. [Google Scholar] [CrossRef]
- Ghaffari, A. Preparation and characterization of free mixed-film of pectin/chitosan/Eudragit® RS intended for sigmoidal drug delivery. Eur. J. Pharm. Biopharm. 2007, 67, 175–186. [Google Scholar] [CrossRef]
- Dichiara, A.B.; Webber, M.R.; Gorman, W.R.; Rogers, R.E. Removal of Copper Ions from Aqueous Solutions via Adsorption on Carbon Nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 15674–15680. [Google Scholar] [CrossRef]
- Ren, X.; Li, J.; Tan, X.; Wang, X. Comparative study of graphene oxide, activated carbon and carbon nanotubes as adsorbents for copper decontamination. Dalton Trans. 2013, 42, 5266–5274. [Google Scholar] [CrossRef]
- Taylor, P.; Afroze, S.; Sen, T.K.; Ang, M.; Nishioka, H. Adsorption of methylene blue dye from aqueous solution by novel biomass Eucalyptus sheathiana bark: Equilibrium, kinetics, thermodynamics and mechanism. Desalin. Water Treat. 2015, 57, 37–41. [Google Scholar]
- Sen, T.K.; Afroze, S.; Ang, H.M. Equilibrium, kinetics and mechanism of removal of methylene blue from aqueous solution by adsorption onto pine cone biomass of Pinus radiata. Water Air Soil Pollut. 2011, 218, 499–515. [Google Scholar] [CrossRef]
- Vadivelan, V.; Vasanth Kumar, K. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Ghodbane, I.; Hamdaoui, O. Removal of mercury (II) from aqueous media using eucalyptus bark: Kinetic and equilibrium studies. J. Hazard. Mater. 2008, 160, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 154, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, H.; Zeng, F.; Li, X.; Sun, J.; Li, C.; Lin, H.; Su, Z. HKUST-1 modified ultrastability cellulose / chitosan composite aerogel for highly efficient removal of methylene blue. Carbohydr. Polym. 2021, 255, 117402. [Google Scholar] [CrossRef]
- Jiang, R.; Fu, Y.-Q.; Zhu, H.-Y.; Yao, J.; Xiao, L. Removal of Methyl Orange from Aqueous Solutions by Magnetic Maghemite/Chitosan Nanocomposite Films: Adsorption Kinetics and Equilibrium. J. Appl. Polym. Sci. 2012, 125, E540–E549. [Google Scholar] [CrossRef]
- Ma, Q.; Shen, F.; Lu, X.; Bao, W.; Ma, H. Studies on the adsorption behavior of methyl orange from dye wastewater onto activated clay. Desalin. Water Treat. 2013, 51, 3700–3709. [Google Scholar] [CrossRef]
- Fumba, G.; Essomba, J.S.; Tagne, G.M.; Nsami, J.N.; Désiré, P.; Bélibi, B.; Mbadcam, J.K. Equilibrium and Kinetic Adsorption Studies of Methyl Orange from Aqueous Solutions Using Kaolinite, Metakaolinite and Activated Geopolymer as Low Cost Adsorbents. J. Acad. Ind. Res. 2014, 3, 156–163. [Google Scholar]
- Zayed, A.M.; Abdel Wahed, M.S.M.; Mohamed, E.A.; Sillanpää, M. Insights on the role of organic matters of some Egyptian clays in methyl orange adsorption: Isotherm and kinetic studies. Appl. Clay Sci. 2018, 166, 49–60. [Google Scholar] [CrossRef]
- Fan, J.; Zhao, Z.; Liu, W.; Xue, Y.; Yin, S. Solvothermal synthesis of different phase N-TiO2and their kinetics, isotherm and thermodynamic studies on the adsorption of methyl orange. J. Colloid Interface Sci. 2016, 470, 229–236. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, X.M.; Wu, M.Y.; Wang, M.M.; Zhao, Y.Y.; Li, T.T. Synthesis and performance characterization of poly(vinyl alcohol)-xanthan gum composite hydrogel. React. Funct. Polym. 2019, 136, 34–43. [Google Scholar] [CrossRef]
- Singha, N.R.; Mahapatra, M.; Karmakar, M.; Dutta, A.; Mondal, H.; Chattopadhyay, P.K. Synthesis of guar gum-: G -(acrylic acid- co -acrylamide- co -3-acrylamido propanoic acid) IPN via in situ attachment of acrylamido propanoic acid for analyzing superadsorption mechanism of Pb(II)/Cd(II)/Cu(II)/MB/MV. Polym. Chem. 2017, 8, 6750–6777. [Google Scholar] [CrossRef]
- Jana, S.; Ray, J.; Bhanja, S.K.; Tripathy, T. Removal of textile dyes from single and ternary solutions using poly(acrylamide-co-N-methylacrylamide) grafted katira gum hydrogel. J. Appl. Polym. Sci. 2018, 135, 1–20. [Google Scholar] [CrossRef]
- Mokhtar, A.; Abdelkrim, S.; Djelad, A.; Sardi, A.; Boukoussa, B.; Sassi, M.; Bengueddach, A. Adsorption behavior of cationic and anionic dyes on magadiite-chitosan composite beads. Carbohydr. Polym. 2020, 229, 115399. [Google Scholar] [CrossRef]
- Rahmi; Ismaturrahmi; Mustafa, I. Methylene blue removal from water using H2SO4 crosslinked magnetic chitosan nanocomposite beads. Microchem. J. 2019, 144, 397–402. [Google Scholar] [CrossRef]
- Lian, F.; Zheng, M.; Chen, M.; Zhu, Y.; Zhang, L.; Zheng, B. Modified xanthan gum for methyl orange uptake: Kinetic, isotherm, and thermodynamic behaviors. Int. J. Biol. Macromol. 2020, 165, 2442–2450. [Google Scholar] [CrossRef]
- Karthika, J.S.; Vishalakshi, B. Novel stimuli responsive gellan gum-graft-poly (DMAEMA) hydrogel as adsorbent for anionic dye. Int. J. Biol. Macromol. 2015, 81, 648–655. [Google Scholar] [CrossRef]
- Preetha, B.K.; Badalamoole, V. Modification of Karaya gum by graft copolymerization for effective removal of anionic dyes. Sep. Sci. Technol. 2019, 54, 2638–2652. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, R.; Wang, J. Adsorption of methyl orange from aqueous solution using chitosan/diatomite composite. Water Sci. Technol. 2017, 75, 1633–1642. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, B.; Xu, J.; Pan, K.; Hou, H.; Hu, J.; Yang, J. Cross-linked chitosan/β-cyclodextrin composite for selective removal of methyl orange: Adsorption performance and mechanism. Carbohydr. Polym. 2018, 182, 106–114. [Google Scholar] [CrossRef]
- Makhado, E.; Pandey, S.; Nomngongo, P.N.; Ramontja, J. Preparation and characterization of xanthan gum-cl-poly (acrylic acid)/o-MWCNTs hydrogel nanocomposite as highly effective re-usable adsorbent for removal of methylene blue from aqueous solutions. J. Colloid Interface Sci. 2018, 513, 700–714. [Google Scholar] [CrossRef]
- Han, S.; Sun, Q.; Zheng, H.; Li, J.; Jin, C. Green and facile fabrication of carbon aerogels from cellulose-based waste newspaper for solving organic pollution. Carbohydr. Polym. 2016, 136, 95–100. [Google Scholar] [CrossRef] [PubMed]













| Langmuir Model | Freundlich Model | ||||||
|---|---|---|---|---|---|---|---|
| qe, exp | q0, cal | KL | R2 | KF | n | R2 | |
| MO | 32.8 | 37.24 | 0.9 | 0.99 | 17.26 | 2.5 | 0.87 |
| MB | 32.6 | 80.58 | 0.1 | 0.91 | 7.89 | 1.27 | 0.99 |
| MB | MO | |||||||
|---|---|---|---|---|---|---|---|---|
| C0 (mg/L) | qe, exp ( mg/g) | qe, cal (mg/g) | K1 (min–1) | R2 | qe, exp (mg/g) | qe, cal (mg/g) | K1 (min–1) | R2 |
| 10 | 6.8 | 1.2 | 0.01 | 0.95 | 7.1 | 2.3 | 0.02 | 0.95 |
| 20 | 13.5 | 4.2 | 0.01 | 0.99 | 14.3 | 6.5 | 0.02 | 0.99 |
| 30 | 19.9 | 5.4 | 0.01 | 0.97 | 21.5 | 15.4 | 0.02 | 0.99 |
| 40 | 26.7 | 9.46 | 0.01 | 0.99 | 27.7 | 18.4 | 0.01 | 0.99 |
| 50 | 32.6 | 6.8 | 0.01 | 0.99 | 32.8 | 19.7 | 0.01 | 0.96 |
| MB | MO | |||||||
|---|---|---|---|---|---|---|---|---|
| C0 (mg/L) | qe, exp (mg/g) | qe, cal (mg/g) | K2 (g mg–1 min–1) | R2 | qe, exp (mg/g) | qe, cal (mg/g) | K2 (g mg–1 min–1) | R2 |
| 10 | 6.8 | 7.21 | 0.06 | 0.99 | 7.1 | 6.98 | 0.04 | 0.99 |
| 20 | 13.5 | 14.85 | 0.02 | 0.99 | 14.3 | 13.9 | 0.02 | 0.99 |
| 30 | 19.9 | 23.3 | 0.006 | 0.99 | 21.5 | 20.4 | 0.01 | 0.99 |
| 40 | 26.7 | 29.8 | 0.004 | 0.99 | 27.7 | 27.6 | 0.008 | 0.99 |
| 50 | 32.6 | 34.81 | 0.003 | 0.99 | 32.8 | 33.3 | 0.009 | 0.99 |
| Dye | Natural Adsorbents | qe (mg/g) | Reference |
|---|---|---|---|
| MB | Polyvinyl alcohol—Xanthan gum hydrogel | 4.16 | [50] |
| Guar gum-g-(acrylic acid-co-acrylamide-co-3-acrylamido propanoic acid) | 27.06 | [51] | |
| poly(acrylamide-co-N-methylacrylamide) grafted katira gum | 21.45 | [52] | |
| chitosan-magadiite hydrogel | 45.25 | [53] | |
| H2SO4 crosslinked magnetic chitosan | 20.408 | [54] | |
| Kg-Ch sponge | 32.6 | Present work | |
| MO | γ-Fe2O3/chitosan composite films | 29.41 | [45] |
| Xanthan gum was modified with acrylamide | 29.56 | [55] | |
| gellan gum-grafted-poly((2-dimethylamino) ethyl methacrylate) | 25.8 | [56] | |
| Karaya gum (KG) with 2-(methacryloyloxyethyl)trimethylammonium chloride | 40.57 | [57] | |
| Chitosan/diatomite composite | 32.12 | [58] | |
| Kg-Ch sponge | 32.8 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
K. Ramakrishnan, R.; Padil, V.V.T.; Wacławek, S.; Černík, M.; Varma, R.S. Eco-Friendly and Economic, Adsorptive Removal of Cationic and Anionic Dyes by Bio-Based Karaya Gum—Chitosan Sponge. Polymers 2021, 13, 251. https://doi.org/10.3390/polym13020251
K. Ramakrishnan R, Padil VVT, Wacławek S, Černík M, Varma RS. Eco-Friendly and Economic, Adsorptive Removal of Cationic and Anionic Dyes by Bio-Based Karaya Gum—Chitosan Sponge. Polymers. 2021; 13(2):251. https://doi.org/10.3390/polym13020251
Chicago/Turabian StyleK. Ramakrishnan, Rohith, Vinod V. T. Padil, Stanisław Wacławek, Miroslav Černík, and Rajender S. Varma. 2021. "Eco-Friendly and Economic, Adsorptive Removal of Cationic and Anionic Dyes by Bio-Based Karaya Gum—Chitosan Sponge" Polymers 13, no. 2: 251. https://doi.org/10.3390/polym13020251