Superior Technique for the Production of Agarose Dressing Containing Sericin and Its Wound Healing Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
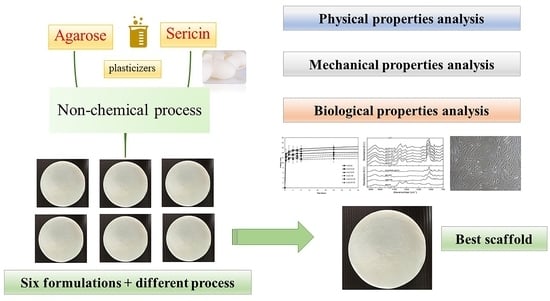
2.2. Scaffold Preparation
2.2.1. Freeze-Drying Method (D)
2.2.2. Freeze-Thawing and Freeze-Drying Method (TD)
2.3. Analysis of Physical Properties
2.3.1. Swelling Properties
2.3.2. Protein Release Profile
2.3.3. Gel Fraction Analysis
2.3.4. Structural Analysis Using Fourier-Transform Infrared Spectroscopy
2.4. Analysis of Mechanical Properties
2.5. Analysis of Biological Properties
2.5.1. Cytotoxic Assay
2.5.2. Cell Migration
2.6. Statistical Analysis
3. Results
3.1. Analysis of Physical Properties
3.1.1. Swelling Properties
3.1.2. Protein Release
3.1.3. Gel Fraction Analysis
3.1.4. Structural Analysis Using Fourier-Transform Infrared Spectroscopy
3.2. Analysis of Mechanical Properties
3.3. Analysis of Biological Properties
3.3.1. Cytotoxic Assay
3.3.2. Cell Migration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Opneja, A.; Kapoor, S.; Stavrou, E.X. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb. Res. 2019, 179, 56–63. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflam. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Guillamat-Prats, R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells 2021, 10, 1729. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Atkin, L. Chronic wounds: The challenges of appropriate management. Br. J. Community Nurs. 2019, 24, S26–S32. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, F.; Momeni-Moghaddam, M.; Naderi-Meshkin, H. Regeneration and Repair of Skin Wounds: Various Strategies for Treatment. Int. J. Low. Extrem. Wounds 2019, 18, 247–261. [Google Scholar] [CrossRef]
- Jia, J.; Duan, S.; Zhou, X.; Sun, L.; Qin, C.; Li, M.; Ge, F. Long-Term Antibacterial Film Nanocomposite Incorporated with Patchouli Essential Oil Prepared by Supercritical CO(2) Cyclic Impregnation for Wound Dressing. Molecules 2021, 26, 5005. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Tan, M.; Zhou, H.; Tang, Y.; Zou, Y. Efficacy of Oxidized Regenerated Cellulose/Collagen Dressing for Management of Skin Wounds: A Systematic Review and Meta-Analysis. Evid. Based Complement. Alternat. Med. 2021, 2021, 1058671. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Fabrication of Hybrid Nanofibers from Biopolymers and Poly (Vinyl Alcohol)/Poly (ε-Caprolactone) for Wound Dressing Applications. Polymers 2021, 13, 2104. [Google Scholar] [CrossRef]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2021, 10, 1–15. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef] [Green Version]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Sherman, J.D.; MacNeill, A.; Thiel, C. Reducing Pollution From the Health Care Industry. JAMA 2019, 322, 1043–1044. [Google Scholar] [CrossRef] [PubMed]
- Kotwani, A.; Joshi, J.; Kaloni, D. Pharmaceutical effluent: A critical link in the interconnected ecosystem promoting antimicrobial resistance. Environ. Sci. Pollut. Res. Int. 2021, 28, 32111–32124. [Google Scholar] [CrossRef] [PubMed]
- Kleywegt, S.; Payne, M.; Ng, F.; Fletcher, T. Environmental loadings of Active Pharmaceutical Ingredients from manufacturing facilities in Canada. Sci. Total Environ. 2019, 646, 257–264. [Google Scholar] [CrossRef]
- Scott, T.M.; Phillips, P.J.; Kolpin, D.W.; Colella, K.M.; Furlong, E.T.; Foreman, W.T.; Gray, J.L. Pharmaceutical manufacturing facility discharges can substantially increase the pharmaceutical load to U.S. wastewaters. Sci. Total Environ. 2018, 636, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Fomo, G.; Madzimbamuto, T.N.; Ojumu, T.V. Applications of Nonconventional Green Extraction Technologies in Process Industries: Challenges, Limitations and Perspectives. Sustainability 2020, 12, 5244. [Google Scholar] [CrossRef]
- Tagliapietra, S.; Binello, A.; Bucciol, F.; Trukhan, V.; Colia, M.; Cravotto, G. Green Enabling Technologies for Competitive Synthesis of Pharmaceutical Lead Compounds. Curr. Pharm. Des. 2020, 26, 5700–5712. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Li, Y.; Huang, S.; Yu, F.; Bei, Y.; Zhang, Y.; Tang, J.; Huang, Y.; Xiang, Q. Hybrid Freeze-Dried Dressings Composed of Epidermal Growth Factor and Recombinant Human-Like Collagen Enhance Cutaneous Wound Healing in Rats. Front. Bioeng. Biotechnol. 2020, 8, 742. [Google Scholar] [CrossRef]
- Ng, S.-F. Freeze-Dried Wafers for Wound Healing. In Therapeutic Dressings and Wound Healing Applications; Boateng, J., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2020; pp. 137–155. [Google Scholar]
- Akiyode, O.; Boateng, J. Composite Biopolymer-Based Wafer Dressings Loaded with Microbial Biosurfactants for Potential Application in Chronic Wounds. Polymers 2018, 10, 918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saporito, F.; Sandri, G.; Rossi, S.; Bonferoni, M.C.; Riva, F.; Malavasi, L.; Caramella, C.; Ferrari, F. Freeze dried chitosan acetate dressings with glycosaminoglycans and traxenamic acid. Carbohydr. Polym. 2018, 184, 408–417. [Google Scholar] [CrossRef]
- Xiao, J.; Zhou, Y.; Ye, M.; An, Y.; Wang, K.; Wu, Q.; Song, L.; Zhang, J.; He, H.; Zhang, Q.; et al. Freeze-Thawing Chitosan/Ions Hydrogel Coated Gauzes Releasing Multiple Metal Ions on Demand for Improved Infected Wound Healing. Adv. Healthc. Mater. 2021, 10, e2001591. [Google Scholar] [CrossRef] [PubMed]
- Lotfipour, F.; Alami-Milani, M.; Salatin, S.; Hadavi, A.; Jelvehgari, M. Freeze-thaw-induced cross-linked PVA/chitosan for oxytetracycline-loaded wound dressing: The experimental design and optimization. Res. Pharm. Sci. 2019, 14, 175–189. [Google Scholar] [PubMed]
- Yang, H.; Tan, Q.; Zhao, H. Progress in various crosslinking modification for acellular matrix. Chin. Med. J. 2014, 127, 3156–3164. [Google Scholar] [PubMed]
- Bhamidipati, M.; Scurto, A.M.; Detamore, M.S. The future of carbon dioxide for polymer processing in tissue engineering. Tissue Eng. Part B Rev. 2013, 19, 221–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Zhang, J.; Yan, J.; Zheng, L. An industrial scale multiple supercritical carbon dioxide apparatus and its eco-friendly dyeing production. J. CO2 Util. 2016, 16, 272–281. [Google Scholar] [CrossRef]
- Kaur, I.P.; Sandhu, S.K.; Deol, P.K.; Sharma, G.; Yadav, M.; Singh, M. Material couture for wound healing and regeneration: An overview. Curr. Pharm. Des. 2015, 21, 1556–1574. [Google Scholar] [CrossRef] [PubMed]
- Naseri-Nosar, M.; Ziora, Z.M. Wound dressings from naturally-occurring polymers: A review on homopolysaccharide-based composites. Carbohydr. Polym. 2018, 189, 379–398. [Google Scholar] [CrossRef]
- Ganesh Saratale, R.; Cho, S.-K.; Dattatraya Saratale, G.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Naresh Bharagava, R.; Kumar, G.; Su Kim, D.; Mulla, S.I.; et al. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef]
- Amini, F.; Semnani, D.; Karbasi, S.; Banitaba, S.N. A novel bilayer drug-loaded wound dressing of PVDF and PHB/Chitosan nanofibers applicable for post-surgical ulcers. Int. J. Polym. Mater. 2019, 68, 772–777. [Google Scholar] [CrossRef]
- Volova, T.; Shishatskaya, E.; Sevastianov, V.; Efremov, S.; Mogilnaya, O. Results of biomedical investigations of PHB and PHB/PHV fibers. Biochem. Eng. J. 2003, 16, 125–133. [Google Scholar] [CrossRef]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef]
- Weller, C.D.; Team, V.; Sussman, G. First-Line Interactive Wound Dressing Update: A Comprehensive Review of the Evidence. Front. Pharmacol. 2020, 11, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.-T. Wound Care Following CO2 Laser Resurfacing Using Kaltostat, Duoderm, and Telfa for Dressings. Dermatol. Surg. 2000, 26, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, H.M.; Aly, A.A.; Sayed, S.M.; Abou-Okeil, A. K-carrageenan/Na-alginate wound dressing with sustainable drug delivery properties. Polym. Adv. Technol. 2021, 32, 1793–1801. [Google Scholar] [CrossRef]
- Normand, V.; Lootens, D.L.; Amici, E.; Plucknett, K.P.; Aymard, P. New insight into agarose gel mechanical properties. Biomacromolecules 2000, 1, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F.; et al. Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Tao, G.; Cai, R.; Wang, P.; Liu, L.; Ai, L.; Zuo, H.; Zhao, P.; Umar, A.; et al. Fabrication of Sericin/Agrose Gel Loaded Lysozyme and Its Potential in Wound Dressing Application. Nanomaterials 2018, 8, 235. [Google Scholar] [CrossRef] [Green Version]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Thönes, S.; Rother, S.; Wippold, T.; Blaszkiewicz, J.; Balamurugan, K.; Moeller, S.; Ruiz-Gómez, G.; Schnabelrauch, M.; Scharnweber, D.; Saalbach, A.; et al. Hyaluronan/collagen hydrogels containing sulfated hyaluronan improve wound healing by sustained release of heparin-binding EGF-like growth factor. Acta Biomater. 2019, 86, 135–147. [Google Scholar] [CrossRef]
- Nurkesh, A.; Jaguparov, A.; Jimi, S.; Saparov, A. Recent Advances in the Controlled Release of Growth Factors and Cytokines for Improving Cutaneous Wound Healing. Front. Cell Dev. Biol. 2020, 8, 638. [Google Scholar] [CrossRef]
- Qi, C.; Xu, L.; Deng, Y.; Wang, G.; Wang, Z.; Wang, L. Sericin hydrogels promote skin wound healing with effective regeneration of hair follicles and sebaceous glands after complete loss of epidermis and dermis. Biomater. Sci. 2018, 6, 2859–2870. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Ding, S.; Shi, W.; Huang, X.; Jiang, L. Bombyx mori silk-based materials with implication in skin repair: Sericin versus regenerated silk fibroin. J. Biomater. Appl. 2019, 34, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Shin, H.-S.; Campos, E.V.R.; Fraceto, L.F.; Del Pilar Rodriguez-Torres, M.; Mariano, K.C.F.; de Araujo, D.R.; Fernández-Luqueño, F.; Grillo, R.; Patra, J.K. Sericin based nanoformulations: A comprehensive review on molecular mechanisms of interaction with organisms to biological applications. J. Nanobiotechnol. 2021, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Napavichayanun, S.; Yamdech, R.; Aramwit, P. The safety and efficacy of bacterial nanocellulose wound dressing incorporating sericin and polyhexamethylene biguanide: In vitro, in vivo and clinical studies. Arch. Dermatol. Res. 2016, 308, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Jantrawut, P.; Chaiwarit, T.; Jantanasakulwong, K.; Brachais, C.H.; Chambin, O. Effect of Plasticizer Type on Tensile Property and In Vitro Indomethacin Release of Thin Films Based on Low-Methoxyl Pectin. Polymers 2017, 9, 289. [Google Scholar] [CrossRef]
- Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The effect of sericin from various extraction methods on cell viability and collagen production. Int. J. Mol. Sci. 2010, 11, 2200–2211. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Zhang, S.; Liu, X.; Ye, S.; Zhou, X.; Huang, Q.; Ren, L. Silk/agarose scaffolds with tunable properties via SDS assisted rapid gelation. RSC Adv. 2017, 7, 21740–21748. [Google Scholar] [CrossRef]
- Felfel, R.M.; Gideon-Adeniyi, M.J.; Zakir Hossain, K.M.; Roberts, G.A.F.; Grant, D.M. Structural, mechanical and swelling characteristics of 3D scaffolds from chitosan-agarose blends. Carbohydr. Polym. 2019, 204, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, F.; Achife, E.C.; Momoda, J.; Shimamura, K.; Monobe, K. Morphology of optically anisotropic agarose hydrogel prepared by directional freezing. Colloid Polym. Sci. 1990, 268, 552–558. [Google Scholar] [CrossRef]
- Neres Santos, A.M.; Duarte Moreira, A.P.; Piler Carvalho, C.W.; Luchese, R.; Ribeiro, E.; McGuinness, G.B.; Fernandes Mendes, M.; Nunes Oliveira, R. Physically Cross-Linked Gels of PVA with Natural Polymers as Matrices for Manuka Honey Release in Wound-Care Applications. Materials 2019, 12, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Wang, C.; Wang, C.; Xiao, Y.; Lin, W. An Overview on Collagen and Gelatin-Based Cryogels: Fabrication, Classification, Properties and Biomedical Applications. Polymers 2021, 13, 2299. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Bakhshandeh, B.; Rezaeian, I.; Heshmatian, B.; Ganjali, M. A Novel Electroactive Agarose-Aniline Pentamer Platform as a Potential Candidate for Neural Tissue Engineering. Sci. Rep. 2017, 7, 17187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurihara, M.; Fukushima, M.; Miyata, A.; Tanaka, T.; Sugimoto, K.; Okada, H. Comparative study of agarose-gel microcapsules and Cryotop in cryopreservation of extremely small numbers of human spermatozoa. Syst. Biol. Reprod. Med. 2021, 67, 244–250. [Google Scholar] [CrossRef]
- Paolicelli, P.; Petralito, S.; Varani, G.; Nardoni, M.; Pacelli, S.; Di Muzio, L.; Tirillò, J.; Bartuli, C.; Cesa, S.; Casadei, M.A.; et al. Effect of glycerol on the physical and mechanical properties of thin gellan gum films for oral drug delivery. Int. J. Pharm. 2018, 547, 226–234. [Google Scholar] [CrossRef]
- Górska, A.; Krupa, A.; Majda, D.; Kulinowski, P.; Kurek, M.; Węglarz, W.P.; Jachowicz, R. Poly(Vinyl Alcohol) Cryogel Membranes Loaded with Resveratrol as Potential Active Wound Dressings. AAPS PharmSciTech 2021, 22, 109. [Google Scholar] [CrossRef]
- Guadarrama-Acevedo, M.C.; Mendoza-Flores, R.A.; Del Prado-Audelo, M.L.; Urbán-Morlán, Z.; Giraldo-Gomez, D.M.; Magaña, J.J.; González-Torres, M.; Reyes-Hernández, O.D.; Figueroa-González, G.; Caballero-Florán, I.H.; et al. Development and Evaluation of Alginate Membranes with Curcumin-Loaded Nanoparticles for Potential Wound-Healing Applications. Pharmaceutics 2019, 11, 389. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.P.; Zhang, Y.; Lang, Y.X.; Yu, M.H. Structural ATR-IR analysis of cellulose fibers prepared from a NaOH complex aqueous solution. IOP Conf. Ser. Mater. Sci. Eng. 2017, 213, 012039. [Google Scholar] [CrossRef] [Green Version]
- Nelson, M.L.; O’Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal latticed type. Part I. Spectra of lattice types I, II, III and of amorphous cellulose. J. Appl. Polym. Sci. 1964, 8, 1311–1324. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, H.-J. Fourier Transform Infrared Spectroscopy (FT-IR) and Simple Algorithm Analysis for Rapid and Non-Destructive Assessment of Developmental Cotton Fibers. Sensors 2017, 17, 1469. [Google Scholar] [CrossRef]
- Kruer-Zerhusen, N.; Cantero-Tubilla, B.; Wilson, D. Characterization of cellulose crystallinity after enzymatic treatment using Fourier transform infrared spectroscopy (FTIR). Cellulose 2018, 25, 37–48. [Google Scholar] [CrossRef]
- Amin, A.; Mohd Sauid, S.; Musa, M.; Hamid, K. The effect of glycerol content on mechanical properties, surface morphology and water absorption of thermoplastic films from tacca leontopetaloides starch. J. Teknol. 2017, 79, 53–59. [Google Scholar]
- Hidayati, S.; Maulidia, U.; Satyajaya, W.; Hadi, S. Effect of glycerol concentration and carboxy methyl cellulose on biodegradable film characteristics of seaweed waste. Heliyon 2021, 7, e07799. [Google Scholar] [CrossRef] [PubMed]
- Arango, M.C.; Montoya, Y.; Peresin, M.S.; Bustamante, J.; Álvarez-López, C. Silk sericin as a biomaterial for tissue engineering: A review. Int. J. Polym. Mater. 2020, 70, 1115–1129. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Borges, S.; Costa-Pinto, A.R.; Costa, R.; Amorim, M.; Dias, J.R.; Ramos, Ó.; Alves, P.; Granja, P.L.; Soares, R.; et al. In Situ Forming Silk Sericin-Based Hydrogel: A Novel Wound Healing Biomaterial. ACS. Biomater. Sci. Eng. 2021, 7, 1573–1586. [Google Scholar] [CrossRef]
- Tao, G.; Cai, R.; Wang, Y.; Zuo, H.; He, H. Fabrication of antibacterial sericin based hydrogel as an injectable and mouldable wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111597. [Google Scholar] [CrossRef]
- Tariq, M.; Tahir, H.M.; Butt, S.A.; Ali, S.; Ahmad, A.B.; Raza, C.; Summer, M.; Hassan, A.; Nadeem, J. Silk derived formulations for accelerated wound healing in diabetic mice. PeerJ 2021, 9, e10232. [Google Scholar] [CrossRef]
- Napavichayanun, S.; Ampawong, S.; Harnsilpong, T.; Angspatt, A.; Aramwit, P. Inflammatory reaction, clinical efficacy, and safety of bacterial cellulose wound dressing containing silk sericin and polyhexamethylene biguanide for wound treatment. Arch. Dermatol. Res. 2018, 310, 795–805. [Google Scholar] [CrossRef]
- Gilotra, S.; Chouhan, D.; Bhardwaj, N.; Nandi, S.K.; Mandal, B.B. Potential of silk sericin based nanofibrous mats for wound dressing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Li, Z.; Gao, C.; Wang, F.; Chen, Z. Preparation and characterization of gelatin/sericin/carboxymethyl chitosan medical tissue glue. J. Appl. Biomater. Funct. Mater. 2018, 16, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Siritientong, T.; Angspatt, A.; Ratanavaraporn, J.; Aramwit, P. Clinical potential of a silk sericin-releasing bioactive wound dressing for the treatment of split-thickness skin graft donor sites. Pharm. Res. 2014, 31, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Tan, S.; Winarto, N.; Dosan, R.; Aisyah, P. The Benefits Of Occlusive Dressings In Wound Healing. Open Dermatol. J. 2019, 13, 27–33. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Przekora, A. Modifications of Wound Dressings with Bioactive Agents to Achieve Improved Pro-Healing Properties. Appl. Sci. 2021, 11, 4114. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2004; p. 366. [Google Scholar]











| Scaffolds | Tensile Strength (MPa) | Elongation (%) | Young’s Modulus (N/mm2) |
|---|---|---|---|
| 3A2S-D | 1.54 ± 0.55 # | 3.53 ± 1.19 | 54.68 ± 11.17 *, # |
| 3A2S1G-D | 1.35 ± 0.14 | 6.76 ± 0.96 * | 29.17 ± 10.44 * |
| 3A2S1P-D | 2.77 ± 0.94 *, # | 4.11 ± 0.86 | 81.43 ± 20.82 *, # |
| 3A2S-TD | 0.51 ± 0.18 | 2.05 ± 0.53 * | 26.92 ± 10.20 |
| 3A2S1G-TD | 0.64 ± 0.13 | 6.13 ± 0.40 * | 13.81 ± 2.62 |
| 3A2S1P-TD | 0.71 ± 0.28 | 3.69 ± 0.56 * | 24.16 ± 6.37 |
| Properties | Ideal Wound Dressing | Dressing in This Study |
|---|---|---|
| No toxic component | Yes | Yes |
| Eco-friendly product | Yes | Yes |
| Lack of cytotoxicity | Yes | Yes |
| Wound healing acceleration | Yes | Yes |
| Wound protection | Yes | Yes |
| Soft elasticity | Yes | Yes |
| Drug delivery | Yes | Yes |
| Excess exudate absorption | Yes | Yes |
| Moist healing environment | Yes | Yes |
| Pain reduction | Yes | Probable |
| Oxygen permeability | Yes | Probable |
| Easy removal | Yes | Probable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napavichayanun, S.; Pienpinijtham, P.; Reddy, N.; Aramwit, P. Superior Technique for the Production of Agarose Dressing Containing Sericin and Its Wound Healing Property. Polymers 2021, 13, 3370. https://doi.org/10.3390/polym13193370
Napavichayanun S, Pienpinijtham P, Reddy N, Aramwit P. Superior Technique for the Production of Agarose Dressing Containing Sericin and Its Wound Healing Property. Polymers. 2021; 13(19):3370. https://doi.org/10.3390/polym13193370
Chicago/Turabian StyleNapavichayanun, Supamas, Prompong Pienpinijtham, Narendra Reddy, and Pornanong Aramwit. 2021. "Superior Technique for the Production of Agarose Dressing Containing Sericin and Its Wound Healing Property" Polymers 13, no. 19: 3370. https://doi.org/10.3390/polym13193370