Chitosan-Coated 5-Fluorouracil Incorporated Emulsions as Transdermal Drug Delivery Matrices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Emulsions
2.3. Physicochemical Characterization of the Emulsions
2.3.1. Size and Zeta-Potential
2.3.2. Morphology of Emulsions
2.3.3. pH of the Emulsions
2.3.4. Viscosity Determination
2.3.5. Emulsification and Phase Separation Study
2.3.6. Drug Loading
2.3.7. Stability Study of the Emulsions
2.3.8. Skin Irritancy Test
2.4. In Vitro Release of the Emulsions
2.5. Drug Release Kinetics
2.6. Ex Vivo Skin Permeation of the Drug
2.7. Skin Drug Retention
2.8. Physicochemical Characterization of the Skin
2.9. Statistical Analysis
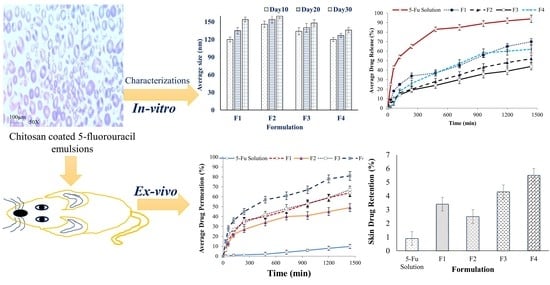
3. Results
3.1. Physicochemical Characterization of the Emulsions
3.1.1. Droplet Size and Zeta-Potential
3.1.2. Drug Loading and % Entrapment Efficiency
3.1.3. Morphology of Emulsions
3.1.4. pH of Emulsions
3.1.5. Viscosity of Emulsions
3.1.6. Ease of Emulsification and the Phase Separation Study
3.1.7. Skin Irritancy Test
3.1.8. Stability Study
3.2. In Vitro Drug Release Study
3.3. Drug Release Kinetics
3.4. In Vitro Permeation Studies
3.5. Skin Drug Retention
3.6. The Physicochemical Characterization of the Skin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Schoellhammer, C.M.; Blankschtein, D.; Langer, R. Skin Permeabilization for Transdermal Drug Delivery: Recent Advances and Future Prospects. Expert Opin. Drug Deliv. 2014, 11, 393–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, T.; Das, D.B. Potential of Combined Ultrasound and Microneedles for Enhanced Transdermal Drug Permeation: A Review. Eur. J. Pharm. Biopharm. 2015, 89, 312–328. [Google Scholar] [CrossRef] [Green Version]
- Lundborg, M.; Wennberg, C.L.; Narangifard, A.; Lindahl, E.; Norlén, L. Predicting drug permeability through skin using molecular dynamics simulation. J. Control. Release 2018, 283, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A.M. Topical pharmacology and toxicology of dimethylsulfoxide. J. Am. Med. Assoc. 1965, 193, 796–804. [Google Scholar] [CrossRef]
- Southwell, D.; Barry, B.W. Penetration enhancers for human skin: Mode of action of 2-Pyrrolidone and dimethylformamide on partition and diffusion of model compound water, n-alcohol and caffeine. J. Investig. Dermatol. 1984, 82, 507–515. [Google Scholar] [CrossRef]
- Park, E.-S.; Chang, S.-J.; Rhee, Y.-S.; Chi, S.-C. Effect of adhesive and permeation enhancer on the skin permeation of captopril. Drug Dev. Ind. Pharm. 2001, 27, 975–980. [Google Scholar] [CrossRef]
- Vollmer, U.; Muller, B.W.; Mesens, J.; Wilffert, B.; Peters, T. In vivo skin pharmacokinetics of liarozole: Percutaneous ahsorption studies with different formulations of cyclodextrin derivatives in rats. Int. J. Pharm. 1993, 99, 51–58. [Google Scholar] [CrossRef]
- Sapra, B.; Jain, S.; Tiwary, A.K. Transdermal delivery of carvedilol containing glycyrrhizin and chitosan as permeation enhancers: Biochemical, biophysical, microscopic and pharmacodynamic evaluation. Drug Deliv. 2008, 15, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Tan, Q.; Liu, W.; Guo, C.; Zhai, G. Preparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical delivery. Int. J. Nanomed. 2011, 6, 1621–1630. [Google Scholar]
- Zhang, Y.-J.; Ma, C.-H.; Lu, W.-L.; Zhang, X.; Wang, X.-L.; Sun, J.-N.; Zhang, Q. Permeation-enhancing effects of chitosan formulations on recombinant hirudin-2 by nasal delivery in vitro and in vivo. Acta Pharmacol. Sin. 2005, 26, 1402–1408. [Google Scholar] [CrossRef] [Green Version]
- Park, S.N.; Jo, N.R.; Jeon, S.H. Chitosan-coated liposomes for enhanced skin permeation of resveratrol. J. Ind. Eng. Chem. 2014, 20, 1481–1485. [Google Scholar] [CrossRef]
- Schipper, N.G.; Olsson, S.; Hoogstraate, J.A.; deBoer, A.G.; Vårum, K.M.; Artursson, P. Chitosan as absorption enhancers for poorly absorbable drugs. 2: Mechanism of absorption enhancement. Pharm. Res. 1997, 14, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Aspden, T.J.; Mason, J.D.; Jones, N.S.; Lowe, J.; Skaugrud, Ø.; Illum, L. Chitosan as a nasal delivery system: The effect of chitosan solutions on in vitro and in vivo mucociliary transport rates in human turbinates and volunteers. J. Pharm. Sci. 1997, 86, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Aung, N.N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Pamornpathomkul, B. Enhancement of transdermal delivery of resveratrol using Eudragit and polyvinyl pyrrolidone-based dissolving microneedle patches. J. Drug Deliv. Sci. Technol. 2021, 61, 102284. [Google Scholar] [CrossRef]
- Malviya, R.; Sundram, S.; Fuloria, S.; Subramaniyan, V.; Sathasivam, K.V.; Azad, A.K.; Fuloria, N.K. Evaluation and Characterization of Tamarind Gum Polysaccharide: The Biopolymer. Polymers 2021, 13, 3023. [Google Scholar] [CrossRef]
- Kotta, S.; Khan, A.W.; Ansari, S.H.; Sharma, R.K.; Ali, J. Formulation of emulsions: A comparison between phase inversion composition method and high-pressure homogenization method. Drug Deliv. 2015, 22, 455–466. [Google Scholar] [CrossRef]
- Rajinikanth, P.S.; Chellian, J. Development and evaluation of nanostructured lipid carrier-based hydrogel for topical delivery of 5-fluorouracil. Int. J. Nanomed. 2016, 11, 5067. [Google Scholar] [CrossRef] [Green Version]
- Naguib, Y.W.; Kumar, A.; Cui, Z. The effect of microneedles on the skin permeability and antitumor activity of topical 5-fluorouracil. Acta Pharm. Sin. B 2014, 4, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Khandavilli, S.; Panchagnula, R. Emulsions as versatile formula-tions for paclitaxel delivery: Peroral and dermal delivery studies in rats. J. Investig. Dermatol. 2007, 127, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Aliaa, N.; ElMeshad, I.; Ibrahim, M.T. Transdermal delivery of an anti-cancer drug via W/O emulsions based on alkyl polyglycosides and lecithin: Design, characterization, and in vivo evaluation of the possible irritation potential in rats. AAPS PharmSciTech 2011, 2, 1–9. [Google Scholar]
- Chaudhari, P.M.; Kuchekar, M.A. Development and evaluation of emulsions as a carrier for topical delivery system by Box-Behnken Design. Asian J. Pharm. Clin. Res. 2018, 11, 286–293. [Google Scholar] [CrossRef]
- Bera, H.; Abbasi, Y.F.; Gajbhiye, V.; Liew, K.F.; Kumar, P.; Tambe, P.; Azad, A.K.; Cuna, D.; Yang, M. Carboxymethyl fenugreek galactomannan-g-poly (N-isopropylacrylamide-co-N, N′-methylene-bis-acrylamide)-clay based pH/temperature-responsive nanocomposites as drug-carriers. Mater. Sci. Eng. C 2020, 110, 110628. [Google Scholar] [CrossRef] [PubMed]
- Parveen, R.; Baboota, S.; Ali, J.; Ahuja, A.; Ahmad, S. Stability studies of silymarin emulsions containing Tween 80 as a surfactant. J. Pharm. Bioallied Sci. 2015, 7, 321. [Google Scholar] [PubMed]
- Baboota, S.; Shakeel, F.; Ahuja, A.; Ali, J.; Shafiq, S. Design, development and evaluation of novel emulsions formulations for transdermal potential of celecoxib. Acta Pharm. 2007, 57, 315–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Costa, S.; Basri, M.; Shamsudin, N.; Basri, H. Stability of Positively Charged Emulsions Formulation Containing Steroidal Drug for Effective Transdermal Application. J. Chem. 2014, 2014, 748680. [Google Scholar] [CrossRef] [Green Version]
- Artiga-Artigas, M.; Lanjari-Pérez, Y.; Martín-Belloso, O. Curcumin-loaded emulsions stability as affected by the nature and concentration of surfactant. Food Chem. 2018, 266, 466–474. [Google Scholar] [CrossRef]
- Akhlaq, M.; Azad, A.K.; Ullah, I.; Nawaz, A.; Safdar, M.; Bhattacharya, T.; Nagaswarupa, H.P. Methotrexate-Loaded Gelatin and Polyvinyl Alcohol (Gel/PVA) Hydrogel as a pH-Sensitive Matrix. Polymers 2021, 13, 2300. [Google Scholar] [CrossRef]
- Tummala, S.; Kumar, M.S.; Prakash, A. Formulation and characterization of 5-Fluorouracil enteric coated nanoparticles for sustained and localized release in treating colorectal cancer. Saudi Pharm. J. 2015, 23, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Jain, S.K. In vitro release kinetics model fitting of liposomes: An insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar] [CrossRef]
- Azad, A.K.; Al-Mahmood, S.M.A.; Kennedy, J.F.; Chatterjee, B.; Bera, H. Electro-hydrodynamic assisted synthesis of lecithin-stabilized peppermint oil-loaded alginate microbeads for intestinal drug delivery. Int. J. Biol. Macromol. 2021, 185, 861–875. [Google Scholar] [CrossRef]
- Lu, T.; Ten Hagen, T.L. A novel kinetic model to describe the ultra-fast triggered release of thermosensitive liposomal drug delivery systems. J. Control. Release 2020, 324, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P.; Martino, M.; Reverchon, E. Supercritical Assisted Production of Lutein-Loaded Liposomes and Modelling of Drug Release. Processes 2021, 9, 1162. [Google Scholar] [CrossRef]
- Azad, A.K.; Al-Mahmood, S.M.A.; Chatterjee, B.; Wan Sulaiman, W.M.A.; Elsayed, T.M.; Doolaanea, A.A. Encapsulation of black seed oil in alginate beads as a ph-sensitive carrier for intestine-targeted drug delivery: In vitro, in vivo and ex vivo study. Pharmaceutics 2020, 12, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilhelm, K.P.; Surber, C.; Maibach, H.I. Effect of sodium lauryl sulfate-induced skin irritation on in vitro percutaneous absorption of four drugs. J. Investig. Dermatol. 1991, 96, 963–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidajat, M.J.; Jo, W.; Kim, H.; Noh, J. Effective Droplet Size Reduction and Excellent Stability of Limonene Emulsions Formed by High-Pressure Homogenizer. Colloids Interfaces 2020, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Su, L.C.; Chen, M.C. Efficient delivery of nanoparticles to deep skin layers using dissolvable microneedles with an extended-length design. J. Mater. Chem. B 2017, 5, 3355–3363. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123, 369–385. [Google Scholar] [CrossRef]
- Wais, M.; Samad, A.; Nazish, I.; Khale, A.; Aqil, M.; Khan, M. Formulation Development Ex-vivo and in-vivo Evaluation of Emulsions for transdermal delivery of glibenclamide. Int. J. Pharm. Pharm. Sci. 2013, 5, 747–754. [Google Scholar]
- Zheng, Y.; Ouyang, W.Q.; Wei, Y.P.; Syed, S.F.; Hao, C.S.; Wang, B.Z.; Shang, Y.H. Effects of carbopol® 934 proportion on emulsions gel for topical and transdermal drug delivery: A skin permeation study. Int. J. Nanomed. 2016, 11, 5971. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, A.; Wong, T.W. Microwave as skin permeation enhancer for transdermal drug delivery of chitosan-5-fluorouracil nanoparticles. Carbohydr. Polym. 2017, 157, 906–919. [Google Scholar] [CrossRef]
- Froebe, C.; Simion, F.; Rhein, L.; Cagan, R.; Kligman, A. Stratum corneum lipid removal by surfactants: Relation to in vivo irritation. Dermatology 1990, 181, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Al-Mahmood, S.M.A.; Doolaanea, A.A. Development and Validation of UV-Vis Spectrophotometric Method for Estimation of Black Seeds and Peppermint Oil in Emulsion. Anal. Chem. Lett. 2021, 11, 607–617. [Google Scholar] [CrossRef]
- Ghafourian, T.; Nokhodchi, A.; Kaialy, W. Surfactants as Penetration Enhancers for Dermal and Transdermal Drug Delivery. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Patel, M.R.; Patel, R.B.; Parikh, J.R. Formulation consideration and skin retention study of microemulsion containing tazarotene for targeted therapy of acne. Int. J. Pharm. Investig. 2016, 46, 55–66. [Google Scholar] [CrossRef]
- Fuloria, N.K.; Fuloria, S. Structural elucidation of small organic molecules by 1D, 2D and multi-dimensional-solution NMR spectroscopy. J. Anal. Bioanal. Tech. 2013, S11, 1–8. [Google Scholar] [CrossRef]







| F. Code | Average Size (nm) | Polydispersity Index | Zeta Potential (mV) |
|---|---|---|---|
| F1 | 120.1 ± 10.52 | 0.281 ± 0.04 | +3.9 ± 0.73 |
| F2 | 141.3 ± 9.31 | 0.326 ± 0.08 | +3.7 ± 0.61 |
| F3 | 129.5 ± 8.92 | 0.272 ± 0.04 | +4.6 ± 0.66 |
| F4 | 109.6 ± 7.23 | 0.241 ± 0.03 | +5.5 ± 0.52 |
| F. Code | pH | Viscosity (cps) | Drug Content | %EE |
|---|---|---|---|---|
| F1 | 5.2 | 17.3 ± 1.32 | 88.5 ± 3.4 | 74.3 ± 2.1 |
| F2 | 5.0 | 29.5 ± 1.22 | 86.9 ± 3.3 | 69.7 ± 3.5 |
| F3 | 5.6 | 16.7 ± 1.13 | 90.7 ± 3.6 | 76.9 ± 2.7 |
| F4 | 5.9 | 18.4 ± 2.09 | 92.1 ± 2.9 | 80.4 ± 3.2 |
| F. Code | No. of Flask Inversions | % Transmittance | Phase Separation | Skin Irritancy | Stability |
|---|---|---|---|---|---|
| F1 | 5 | 85.53 | No | No | Stable |
| F2 | 7 | 79.20 | No | Yes | Stable |
| F3 | 6 | 87.91 | No | No | Stable |
| F4 | 4 | 97.7 | No | No | Stable |
| F. Code | b | R2 |
|---|---|---|
| F1 | 0.643 ± 0.54 | 0.695 ± 0.47 |
| F2 | 0.850 ± 0.43 | 0.755 ± 0.42 |
| F3 | 0.767 ± 0.79 | 0.789 ± 0.92 |
| F4 | 0.897 ± 0.16 | 0.886 ± 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, T.A.; Azad, A.K.; Fuloria, S.; Nawaz, A.; Subramaniyan, V.; Akhlaq, M.; Safdar, M.; Sathasivam, K.V.; Sekar, M.; Porwal, O.; et al. Chitosan-Coated 5-Fluorouracil Incorporated Emulsions as Transdermal Drug Delivery Matrices. Polymers 2021, 13, 3345. https://doi.org/10.3390/polym13193345
Khan TA, Azad AK, Fuloria S, Nawaz A, Subramaniyan V, Akhlaq M, Safdar M, Sathasivam KV, Sekar M, Porwal O, et al. Chitosan-Coated 5-Fluorouracil Incorporated Emulsions as Transdermal Drug Delivery Matrices. Polymers. 2021; 13(19):3345. https://doi.org/10.3390/polym13193345
Chicago/Turabian StyleKhan, Taif Ali, Abul Kalam Azad, Shivkanya Fuloria, Asif Nawaz, Vetriselvan Subramaniyan, Muhammad Akhlaq, Muhammad Safdar, Kathiresan V. Sathasivam, Mahendran Sekar, Omji Porwal, and et al. 2021. "Chitosan-Coated 5-Fluorouracil Incorporated Emulsions as Transdermal Drug Delivery Matrices" Polymers 13, no. 19: 3345. https://doi.org/10.3390/polym13193345