Study of Different Chitosan/Sodium Carboxymethyl Cellulose Proportions in the Development of Polyelectrolyte Complexes for the Sustained Release of Clarithromycin from Matrix Tablets
Abstract
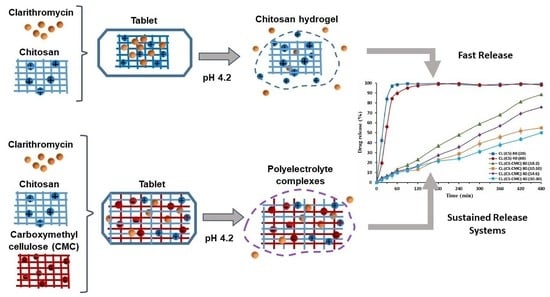
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Formulations
2.2.2. Scanning Electron Microscopy (SEM) Studies
2.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.4. Differential Scanning Calorimetry (DSC)
2.2.5. Powder X-ray Diffraction (PXRD)
2.2.6. In Vitro Drug Release
3. Results and Discussion
3.1. Scanning Electron Microscopy (SEM) Characterization
3.2. FTIR Spectroscopy Analysis
3.3. DSC Studies
3.4. Powder X-ray Diffraction (PXRD)
3.5. In Vitro Drug Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eliyahu, S.; Galitsky, A.; Ritov, E.; Bianco-Peled, H. Hybrid Acrylated Chitosan and Thiolated Pectin Cross-Linked Hydrogels with Tunable Properties. Polymers 2021, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Iijima, K. Fabrication and Characterization of Polysaccharide Composite Films from Polyion Complex Particles. Polymers 2020, 12, 435. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Ji, Y.; Sun, Q.; Fu, Y.; Xu, Y.; Jin, L. Fabrication of Cellulose Nanocrystal/Chitosan Hydrogel for Controlled Drug Release. Nanomaterials 2019, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Chaves de Souza, M.P.; de Mattos, N.H.; Pedreiro, L.N.; Boni, F.I.; dos Santos Ramos, M.A.; Bauab, T.M.; Daflon Germiao, M.P.; Chorilli, M. Design of Mucoadhesive Nanostructured Polyelectrolyte Complexes Based on Chitosan and Hypromellose Phthalate for Metronidazole Delivery Intended to the Treatment of Helicobacter pylori Infections. Pharmaceutics 2020, 12, 1211. [Google Scholar] [CrossRef]
- Ahuja, M.; Bhatt, D.C. Polyelectrolyte complex of carboxymethyl gum katira-chitosan: Preparation and characterization. Int. J. Biol. Macromol. 2018, 106, 1184–1191. [Google Scholar] [CrossRef]
- Jin, L.; Qi, H.; Gu, X.; Zhang, X.; Zhang, Y.; Zhang, X.; Mao, S. Effect of Sodium Alginate Type on Drug Release from Chitosan-Sodium Alginate-Based In Situ Film-Forming Tablets. AAPS PharmSciTech 2020, 21, 55. [Google Scholar] [CrossRef] [PubMed]
- Abu Fara, D.; Dadou, S.M.; Rashid, I.; Al-Obeidi, R.; Antonijevic, M.D.; Chowdhry, B.Z.; Badwan, A. A direct compression matrix made from xanthan gum and low molecular weight chitosan designed to improve compressibility in controlled release tablets. Pharmaceutics 2019, 11, 603. [Google Scholar] [CrossRef] [Green Version]
- Ćirić, A.; Medarević, D.; Čalija, B.; Dobričić, V.; Mitrić, M.; Djekic, L. Study of chitosan/xanthan gum polyelectrolyte complexes formation, solid state and influence on ibuprofen release kinetics. Int. J. Biol. Macromol. 2020, 148, 942–955. [Google Scholar] [CrossRef]
- Roy, J.C.; Ferri, A.; Giraud, S.; Jinping, G.; Salaün, F. Chitosan–carboxymethylcellulose-based polyelectrolyte complexation and microcapsule shell formulation. Int. J. Mol. Sci. 2018, 19, 2521. [Google Scholar] [CrossRef] [Green Version]
- Fabiano, A.; Beconcini, D.; Migone, C.; Piras, A.M.; Zambito, Y. Quaternary ammonium chitosans: The importance of the positive fixed charge of the drug delivery systems. Int. J. Mol. Sci. 2020, 21, 6617. [Google Scholar] [CrossRef]
- Soisuwan, S.; Teeranachaideekul, V.; Wongrakpanich, A.; Langguth, P.; Junyaprasert, V.B. Impact of uncharged and charged stabilizers on in vitro drug performances of clarithromycin nanocrystals. Eur. J. Pharm. Biopharm. 2019, 137, 68–76. [Google Scholar] [CrossRef]
- Graham, D.Y.; Tansel, A. Interchangeable use of proton pump inhibitors based on relative potency. Clin. Gastroenterol. Hepatol. 2018, 16, 800–808. [Google Scholar] [CrossRef]
- Li, N.; Zhong, Q. Effects of polysaccharide charge density on the structure and stability of carboxymethylcellulose-casein nanocomplexes at pH 4.5 prepared with and without a pH-cycle. Food Hydrocoll 2021, 106718. [Google Scholar] [CrossRef]
- Manniello, M.D.; Del Gaudio, P.; Porta, A.; Aquino, R.P.; Russo, P. Aerodynamic properties, solubility and in vitro antibacterial efficacy of dry powders prepared by spray drying: Clarithromycin versus its hydrochloride salt. Eur. J. Pharm. Biopharm. 2016, 104, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Gharekhani, A.; Hamishehkar, H.; Zakeri-Milani, P.; Gharekhani, H. Stomach-specific drug delivery of clarithromycin using a semi interpenetrating polymeric network hydrogel made of montmorillonite and chitosan: Synthesis, characterization and in vitro drug release study. Adv. Pharm. Bull. 2019, 9, 159. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Mohammed, A.A.; Ahmad, J.; Saleh, E. Development of a 3D printed coating shell to control the drug release of encapsulated immediate-release tablets. Polymers 2020, 12, 1395. [Google Scholar] [CrossRef] [PubMed]
- Unagolla, J.M.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Fernandez-Gutierrez, M.; San Roman, J.; Mijangos, C.; Hernández, R. Local and controlled release of tamoxifen from multi (layer-by-layer) alginate/chitosan complex systems. Carbohydr. Polym. 2019, 206, 428–434. [Google Scholar] [CrossRef]
- Ata, S.; Rasool, A.; Islam, A.; Bibi, I.; Rizwan, M.; Azeem, M.K.; Iqbal, M. Loading of Cefixime to pH sensitive chitosan based hydrogel and investigation of controlled release kinetics. Int. J. Biol. Macromol. 2020, 155, 1236–1244. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Tang, X.; Huang, K.; Chen, L. Polyelectrolyte three layer nanoparticles of chitosan/dextran sulfate/chitosan for dual drug delivery. Colloids Surf. B Biointerfaces 2020, 190, 110925. [Google Scholar] [CrossRef]
- Ponsubha, S.; Jaiswal, A.K. Effect of interpolymer complex formation between chondroitin sulfate and chitosan-gelatin hydrogel on physico-chemical and rheological properties. Carbohydr. Polym. 2020, 238, 116179. [Google Scholar] [CrossRef]
- Wu, Q.X.; Wang, Z.D.; Zheng, M.F.; Su, T.; Wang, X.H.; Guan, Y.X.; Chen, Y. Development of metformin hydrochloride loaded dissolving tablets with novel carboxymethylcellulose/poly-l-lysine/TPP complex. Int. J. Biol. Macromol. 2020, 155, 411–420. [Google Scholar] [CrossRef]
- Al-Zoubi, N.; Odah, F.; Obeidat, W.; Al-Jaberi, A.; Partheniadis, I.; Nikolakakis, I. Evaluation of spironolactone solid dispersions prepared by co-spray drying with soluplus® and polyvinylpyrrolidone and influence of tableting on drug release. J. Pharm. Sci. 2018, 107, 2385–2398. [Google Scholar] [CrossRef]
- Ren, T.; Lin, X.; Zhang, Q.; You, D.; Liu, X.; Tao, X.; Tang, X. Encapsulation of azithromycin ion pair in liposome for enhancing ocular delivery and therapeutic efficacy on dry eye. Mol. Pharm. 2018, 15, 4862–4871. [Google Scholar] [CrossRef]
- Li, Z.; Kuang, H.; Yang, J.; Hu, J.; Ding, B.; Sun, W.; Luo, Y. Improving emulsion stability based on ovalbumin-carboxymethyl cellulose complexes with thermal treatment near ovalbumin isoelectric point. Sci. Rep. 2020, 10, 3456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, B.P.; Tsianou, M. From Polyelectrolyte Complexes to Polyelectrolyte Multilayers: Electrostatic Assembly, Nanostructure, Dynamics, and Functional Properties. Adv. Colloid Interface Sci. 2017, 244, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Shiba, R.; Watanabe, M.; Iwao, Y.; Itai, S.; Noguchi, S. Phase transitions of antibiotic clarithromycin forms I, IV and new form VII crystals. Int. J. Pharm. 2018, 547, 258–264. [Google Scholar] [CrossRef]
- Soisuwan, S.; Teeranachaideekul, V.; Wongrakpanich, A.; Langguth, P.; Junyaprasert, V.B. In vitro performances and cellular uptake of clarithromycin nanocrystals produced by media milling technique. Powder Technol. 2018, 338, 471–480. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Feitosa, J.P.A.; Andrade, F.K.; Miranda, M.A.R.; Sasaki, J.M.; Morais, J.P.S.; Alexandre e Silva, L.M.; Marques Canuto, K.; Freitas Rosa, M. Chemically modified cellulose nanocrystals as polyanion for preparation of polyelectrolyte complex. Cellulose 2019, 26, 1725–1746. [Google Scholar] [CrossRef]
- Hanafy, A.F.; Abdalla, A.M.; Guda, T.K.; Gabr, K.E.; Royall, P.G.; Alqurshi, A. Ocular anti-inflammatory activity of prednisolone acetate loaded chitosan-deoxycholate self-assembled nanoparticles. Int. J. Nanomed. 2019, 14, 3679. [Google Scholar] [CrossRef] [Green Version]
- Lal, N.; Dubey, J.; Gaur, P.; Verma, N.; Verma, A. Chitosan based in situ forming polyelectrolyte complexes: A potential sustained drug delivery polymeric carrier for high dose drugs. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, E.G.; De Caland, L.B.; De Medeiros, A.S.; Fernandes-Pedrosa, M.F.; Soares-Sobrinho, J.L.; Dos Santos, K.S.; Antonio, A. Tailoring drug release properties by gradual changes in the particle engineering of polysaccharide chitosan based powders. Polymers 2017, 9, 253. [Google Scholar] [CrossRef] [Green Version]
- Leonida, M.; Ispas-Szabo, P.; Mateescu, M.A. Self-stabilized chitosan and its complexes with carboxymethyl starch as excipients in drug delivery. Bioact. Mater. 2018, 3, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Torrado-Salmerón, C.; Guarnizo-Herrero, V.; Gallego-Arranz, T.; del Val-Sabugo, Y.; Torrado, G.; Morales, J.; Torrado-Santiago, S. Improvement in the Oral Bioavailability and Efficacy of New Ezetimibe Formulations. Comparative Study of a Solid Dispersion and Different Micellar Systems. Pharmaceutics 2020, 12, 617. [Google Scholar] [CrossRef] [PubMed]
- Consumi, M.; Leone, G.; Pepi, S.; Tamasi, G.; Lamponi, S.; Donati, A.; Magnani, A. Xanthan Gum–Chitosan: Delayed, prolonged, and burst-release tablets using same components in different ratio. Adv. Polymer Tech. 2018, 37, 2936–2945. [Google Scholar] [CrossRef]






| Formulations | n | R2 |
|---|---|---|
| CL:(CS:CMC) 80:(18:2) | 0.8696 | 0.9853 |
| CL:(CS:CMC) 40:(54:6) | 0.8613 | 0.9855 |
| CL:(CS:CMC) 80:(10:10) | 0.8611 | 0.9891 |
| CL:(CS:CMC) 40:(30:30) | 0.8108 | 0.9961 |
| Formulations | Kinetic Models | K | R2 |
|---|---|---|---|
| CL:(CS:CMC) 80:(18:2) | Zero-order | 0.0019 | 0.9983 |
| Higuchi | 0.0412 | 0.9629 | |
| First-order | 0.0084 | 0.9134 | |
| CL:(CS:CMC) 40:(54:6) | Zero-order | 0.0015 | 0.9944 |
| Higuchi | 0.0348 | 0.9465 | |
| First-order | 0.0070 | 0.9193 | |
| CL:(CS:CMC) 80:(10:10) | Zero-order | 0.0012 | 0.9846 |
| Higuchi | 0.0410 | 0.8634 | |
| First-order | 0.0056 | 0.8672 | |
| CL:(CS:CMC) 40:(30:30) | Zero-order | 0.0010 | 0.9949 |
| Higuchi | 0.0254 | 0.9717 | |
| First-order | 0.0052 | 0.8477 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarnizo-Herrero, V.; Torrado-Salmerón, C.; Torres Pabón, N.S.; Torrado Durán, G.; Morales, J.; Torrado-Santiago, S. Study of Different Chitosan/Sodium Carboxymethyl Cellulose Proportions in the Development of Polyelectrolyte Complexes for the Sustained Release of Clarithromycin from Matrix Tablets. Polymers 2021, 13, 2813. https://doi.org/10.3390/polym13162813
Guarnizo-Herrero V, Torrado-Salmerón C, Torres Pabón NS, Torrado Durán G, Morales J, Torrado-Santiago S. Study of Different Chitosan/Sodium Carboxymethyl Cellulose Proportions in the Development of Polyelectrolyte Complexes for the Sustained Release of Clarithromycin from Matrix Tablets. Polymers. 2021; 13(16):2813. https://doi.org/10.3390/polym13162813
Chicago/Turabian StyleGuarnizo-Herrero, Víctor, Carlos Torrado-Salmerón, Norma Sofía Torres Pabón, Guillermo Torrado Durán, Javier Morales, and Santiago Torrado-Santiago. 2021. "Study of Different Chitosan/Sodium Carboxymethyl Cellulose Proportions in the Development of Polyelectrolyte Complexes for the Sustained Release of Clarithromycin from Matrix Tablets" Polymers 13, no. 16: 2813. https://doi.org/10.3390/polym13162813