Biosynthesis of Bacterial Cellulose by Extended Cultivation with Multiple Removal of BC Pellicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Inoculum Preparation
2.2. Preparation of Nutrient Media
2.3. Fermentation
2.4. Purification of BC
2.5. Calculation of BC Yield
2.6. Analytical Techniques
2.7. Prosthetic Tension-Free Hernioplasty
3. Results and Discussion
3.1. The Number of BC Pellicle Removals
3.2. Glucose Concentration and BC Yield
3.3. BC Properties
3.4. Outcomes of Tension-Free Hernioplasty
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the fermentation process and properties of bacterial cellulose: A review. Cellulose 2015, 23, 57–91. [Google Scholar] [CrossRef]
- Keshk, S.M.A.S. Bacterial Cellulose production and its industrial applications. J. Bioprocess. Biotech. 2014, 4, 150–160. [Google Scholar] [CrossRef]
- Volova, T.G.; Shumilova, A.A.; Shidlovskiy, I.P.; Nikolaeva, E.D.; Sukovatiy, A.G.; Vasiliev, A.D.; Shishatskay, E.I. Antibacterial properties of films of cellulose composites with silver nanoparticles and antibiotics. Polym. Test. 2018, 65, 54–68. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods–A review. Carbohydr. Polym. 2019, 219, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Zahan, K.A.; Azizul, N.M.; Mustapha, M.; Tong, W.Y.; Rahman, M.S.A.; Sahuri, I.S. Application of bacterial cellulose film as a biodegradable and antimicrobial packaging material. Mater. Today-Proc. 2020, 31, 83–88. [Google Scholar] [CrossRef]
- Yin, N.; Du, R.; Zhao, F.; Han, Y.; Zhou, Z. Characterization of antibacterial bacterial cellulose composite membranes modified with chitosan or chitooligosaccharide. Carbohydr. Polym. 2020, 229, 115520. [Google Scholar] [CrossRef]
- Abeer, M.M.; Amin, M.C.I.M.; Martin, C. A review of bacterial cellulose-based drug delivery systems: Their biochemistry, current approaches and future prospects. J. Pharm. Pharmacol. 2014, 66, 1047–1061. [Google Scholar] [CrossRef]
- Barud, H.G.O.; da Silva, R.R.; Barud, H.S.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; de Oliveira, O.B.; Ribeiro, S.J.L. A multipurpose natural and renewable polymer in medical applications: Bacterial cellulose. Carbohydr. Polym. 2016, 153, 406–420. [Google Scholar] [CrossRef] [Green Version]
- Gama, M.; Dourado, F.; Bielecki, S. Bacterial NanoCellulose from Biotechnology to Bio-Economy; Elsevier: Amsterdam, The Netherlands, 2016; p. 260. [Google Scholar]
- Moniri, M.; Moghaddam, A.B.; Azizi, S.; Rahim, R.A.; Ariff, A.B.; Saad, W.Z.; Navaderi, M.; Mohamad, R. Production and status of bacterial cellulose in biomedical engineering. Nanomaterials 2017, 7, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef] [PubMed]
- Zharikov, A.N.; Lubyansky, V.G.; Gladysheva, E.K.; Skiba, E.A.; Budaeva, V.V.; Semyonova, E.N.; Zharikov, A.A.; Sakovich, G.V. Early morphological changes in tissues when replacing abdominal wall defects by bacterial nanocellulose in experimental trials. J. Mater. Sci. Mater. Med. 2018, 29, 95. [Google Scholar] [CrossRef]
- Nagashima, A.; Tsuji, T.; Kondo, T. A uniaxially oriented nanofibrous cellulose scaffold from pellicles produced by Gluconacetobacter xylinus in dissolved oxygen culture. Carbohydr. Polym. 2016, 135, 215–224. [Google Scholar] [CrossRef]
- Pillai, M.M.; Tran, H.N.; Sathishkumar, G.; Manimekalai, K.; Yoon, J.H.; Lim, D.Y.; Noh, I.; Bhattacharyya, A. Symbiotic culture of nanocellulose pellicle: A potential matrix for 3D Bioprinting. Mater. Sci. Eng. C 2021, 119, 111552. [Google Scholar] [CrossRef]
- Römling, U.; Galperin, M.Y. Bacterial cellulose biosynthesis: Diversity of operons, subunits, products, and functions. Trends Microbiol. 2015, 23, 545–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from industrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Skiba, E.A.; Budaeva, V.V.; Ovchinnikova, E.V.; Gladysheva, E.K.; Kashcheyeva, E.I.; Pavlov, I.N.; Sakovich, G.V. A technology for pilot production of bacterial cellulose from oat hulls. Chem. Eng. Technol. 2020, 383, 123128. [Google Scholar] [CrossRef]
- Skiba, E.A.; Gladysheva, E.K.; Golubev, D.S.; Budaeva, V.V.; Aleshina, L.A.; Sakovich, G.V. Self-standardization of quality of bacterial cellulose produced by Medusomyces gisevii in nutrient media derived from Miscanthus biomass. Carbohydr. Polym. 2021, 252, 117178. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Riaño, M.; Bojacá, V. Production of bacterial cellulose from alternative low-cost substrates. Cellulose 2017, 24, 2677–2698. [Google Scholar] [CrossRef]
- Krystynowicz, A.; Czaja, W.; Wiktorowska-Jezierska, A.; Gonçalves-Miśkiewicz, M.; Turkiewicz, M.; Bielecki, S. Factors affecting the yield and properties of bacterial cellulose. J. Ind. Microbiol. Biotechnol. 2002, 29, 189–195. [Google Scholar] [CrossRef]
- Sani, A.; Dahman, Y. Improvements in the production of bacterial synthesized biocellulose nanofibres using different culture methods. J. Chem. Technol. Biotechnol. 2010, 85, 151–164. [Google Scholar] [CrossRef]
- Watanabe, K.; Tabuchi, M.; Morinaga, Y.; Yoshinaga, F. Structural features and properties of bacterial cellulose produced in agitated culture. Cellulose 1998, 5, 187–200. [Google Scholar] [CrossRef]
- Skiba, E.A.; Baibakova, O.V.; Gladysheva, E.K.; Budaeva, V.V. Study of the influence of Medusomyces gisevii Sa-12 inoculum dosage on bacterial cellulose yield and degree of polymerisation. Izv. Vuzov. Prikl. Khimiya i Biotekhnologiya (Proc. Univ. Appl. Chem. Biotechnol.) 2019, 9, 420–429. [Google Scholar] [CrossRef] [Green Version]
- Hornung, M.; Ludwig, M.; Gerrard, A.M.; Schmauder, H.P. Optimizing the production of bacterial cellulose in surface culture: Evaluation of substrate mass transfer influences on the bioreaction (Part 1). Eng. Life Sci. 2006, 6, 537–545. [Google Scholar] [CrossRef]
- Hornung, M.; Ludwig, M.; Gerrard, A.M.; Schmauder, H.P. Optimizing the production of bacterial cellulose in surface culture: Evaluation of product movement influences on the bioreaction (Part 2). Eng. Life Sci. 2006, 6, 546–551. [Google Scholar] [CrossRef]
- Gromovykh, T.I.; Pigaleva, M.A.; Gallyamov, M.O.; Ivanenko, I.P.; Ozerova, K.E.; Kharitonova, E.P.; Bahman, M.; Feldman, N.B.; Lutsenko, S.V.; Kiselyova, O.I. Structural organization of bacterial cellulose: The origin of anisotropy and layered structures. Carbohydr. Polym. 2020, 237, 116140. [Google Scholar] [CrossRef] [PubMed]
- Yassine, F.; Bassil, N.; Chokr, A.; El Samrani, A.; Serghei, A.; le Boiteux, G.; El Tahchi, M. Two-step formation mechanism of Acetobacter cellulosic biofilm: Synthesis of sparse and compact cellulose. Cellulose 2016, 23, 1087–1100. [Google Scholar] [CrossRef]
- Borzani, W.; Souza, S.J. Mechanism of the film thickness increasing during the bacterial production of cellulose on non-agitated liquid media. Biotechnol. Lett. 1995, 17, 1271–1272. [Google Scholar] [CrossRef]
- Dubey, S.; Singh, J.; Singh, R.P. Biotransformation of sweet lime pulp waste into high-quality nanocellulose with an excellent productivity using Komagataeibacter europaeus SGP37 under static intermittent fed-batch cultivation. Bioresour. Technol. 2018, 247, 73–80. [Google Scholar] [CrossRef]
- Hornung, M.; Ludwig, M.; Schmauder, H.P. Optimizing the production of bacterial cellulose in surface culture: A novel aerosol bioreactor working on a fed batch principle (Part 3). Eng. Life Sci. 2007, 7, 35–41. [Google Scholar] [CrossRef]
- Gladysheva, E.K.; Skiba, E.A.; Zolotukhin, V.N.; Sakovich, G.V. Study of the conditions for the biosynthesis of bacterial cellulose by the producer Medusomyces gisevii Sa-12. Appl. Biochem. Microbiol. 2018, 54, 179–187. [Google Scholar] [CrossRef]
- Monod, J. The growth of bacterial cultures. Annu. Rev. Microbiol. 1949, 3, 371–394. [Google Scholar] [CrossRef] [Green Version]
- Stepanov, N.; Efremenko, E. “Deceived” concentrated immobilized cells as biocatalyst for intensive bacterial cellulose production from various sources. Catalysts 2018, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-based analysis of the bacterial and fungal Compositions of multiple kombucha (tea fungus) samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Yurkevich, D.I.; Kutyshenko, V.P. Medusomyces (Tea fungus): A scientific history, composition, features of physiology and metabolism. Biophysics 2002, 47, 1035–1048. [Google Scholar]
- Goh, W.N.; Rosma, A.; Kaur, B.; Fazilah, A.; Karim, A.A.; Rajeev, B. Fermentation of black tea broth (Kombucha): I. Effects of sucrose concentration and fermentation time on the yield of microbial cellulose. Int. Food Res. J. 2012, 19, 109–117. [Google Scholar]
- Budaeva, V.V.; Gismatulina, Y.A.; Mironova, G.F.; Skiba, E.A.; Gladysheva, E.K.; Kashcheyeva, E.I.; Baibakova, O.V.; Korchagina, A.A.; Shavyrkina, N.A.; Golubev, D.S.; et al. Bacterial Nanocellulose Nitrates. Nanomaterials 2019, 9, 1694. [Google Scholar] [CrossRef] [Green Version]
- Hestrin, S.; Schramm, M. Synthesis of cellulose by Acetobacter xylinum: II. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Abol-Fotouh, D.; Hassan, M.A.; Shokry, H.; Roig, A.; Azab, M.S.; Kashyout, A.B. Bacterial nanocellulose from agro-industrial wastes: Low-cost and enhanced production by Komagataeibacter saccharivorans MD1. Sci. Rep. 2020, 10, 3419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bogolitsyn, K.; Parshina, A.; Aleshina, L. Structural features of brown algae cellulose. Cellulose 2020, 27, 9787–9800. [Google Scholar] [CrossRef]
- Chen, G.; Wu, G.; Chen, L.; Wang, W.; Hong, F.F.; Jönsson, L.J. Performance of nanocellulose-producing bacterial strains in static and agitated cultures with different starting pH. Carbohydr. Polym. 2019, 215, 280–288. [Google Scholar] [CrossRef]
- Chen, G.; Wu, G.; Chen, L.; Wang, W.; Hong, F.F.; Jönsson, L.J. Comparison of productivity and quality of bacterial nanocellulose synthesized using culture media based on seven sugars from biomass. Microb. Biotechnol. 2019, 12, 677–687. [Google Scholar] [CrossRef] [Green Version]
- Kutyshenko, V.P.; Yurkevich, D.I. Influence of heavy water on the metabolism of a symbiotic organism. Biophysics 2003, 48, 648–656. [Google Scholar]
- Bikales, N.M.; Segal, L. Cellulose and Cellulose Derivatives, Vols. IV-V; Wiley Intersciense: New York, NY, USA, 1971; p. 510. [Google Scholar]
- Shi, Q.S.; Feng, J.; Li, W.R.; Zhou, G.; Chen, A.M.; Ouyang, Y.S.; Chen, Y.B. Effect of different conditions on the average degree of polymerization of bacterial cellulose produced by Gluconacetobacter Intermedius BC-41. Cellul. Chem. Technol. 2013, 47, 503–508. [Google Scholar]
- Molina-Ramírez, C.; Cañas-Gutiérrez, A.; Castro, C.; Zuluaga, R.; Gañán, P. Effect of production process scale-up on the characteristics and properties of bacterial nanocellulose obtained from overripe Banana culture medium. Carbohydr. Polym. 2020, 240, 116341. [Google Scholar] [CrossRef]
- Orlovska, I.; Podolich, O.; Kukharenko, O.; Zaets, I.; Reva, O.; Khirunenko, L.; Zmejkoski, D.; Rogalsky, S.; Barh, D.; Tiwari, S.; et al. Bacterial cellulose retains robustness but its synthesis declines after exposure to a Mars-like environment simulated outside the International Space Station. Astrobiology 2021, 21, 706–717. [Google Scholar] [CrossRef] [PubMed]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- French, A.D.; Cintron, M.S. Cellulose polymorphy, crystallite size, and the Segal Crystallinity Index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]
- Podolich, O.; Zaets, I.; Kukharenko, O.; Orlovska, I.; Reva, O.; Khirunenko, L.; Sosnin, M.; Haidak, A.; Shpylova, S.; Rabbow, E.; et al. Kombucha multimicrobial community under simulated spaceflight and Martian conditions. Astrobiology 2017, 17, 459–469. [Google Scholar] [CrossRef]
- Podolich, O.; Kukharenko, O.; Haidak, A.; Zaets, I.; Zaika, L.; Storozhuk, O.; Palchikovska, L.; Orlovska, I.; Reva, O.; Borisova, T.; et al. Multimicrobial Kombucha vulture tolerates Mars-like conditions simulated on low-Earth orbit. Astrobiology 2019, 19, 183–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, J.W.A.; Halm, J.A.; Wijsmuller, A.R.; ten Raa, S.; Jeekel, S.J. Evaluation of new prosthetic meshes for ventral hernia repair. Surg. Endosc. 2006, 20, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, A.; Matone, J.; Marcondes, W.; Herbella, F.A.M.; de Mattos Farah, J.F. Comparative study of inflammatory response and adhesions formation after fixation of different meshes for inguinal hernia repair in rabbits. Acta Cir. Bras. 2005, 20, 347–352. [Google Scholar] [CrossRef] [Green Version]
- Gerullis, H.; Georgas, E.; Borós, M.; Klosterhalfen, B.; Eimer, C.; Arndt, C.; Otto, S.; Barski, D.; Ysebaert, D.; Ramon, A.; et al. Inflammatory reaction as determinant of foreign body reaction is an early and susceptible event after mesh implantation. BioMed Res. Int. 2014, 2014, 510807. [Google Scholar] [CrossRef]
- Klinge, U.; Dietz, U.; Fet, N.; Klosterhalfen, B. Characterisation of the cellular infiltrate in the foreign body granuloma of textile meshes with its impact on collagen deposition. Hernia 2014, 18, 571–578. [Google Scholar] [CrossRef]
- Vaz, M.; Krebs, R.K.; Trindade, E.N.; Trindade, M.R. Fibroplasia after polypropylene mesh implantation for abdominal wall hernia repair in rats. Acta Cir. Bras. 2009, 24, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weyhe, D.; Belyaev, O.; Müller, C.; Meurer, K.; Bauer, K.H.; Papapostolou, G.; Uhl, W. Improving outcomes in hernia repair by the use of light meshes a comparison of different implant constructions based on a critical appraisal of the literature. World J. Surg. 2007, 31, 234–244. [Google Scholar] [CrossRef]





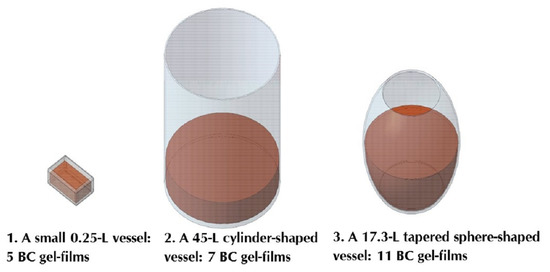
| Option | 1 | 2 | 3 |
|---|---|---|---|
| Vessel material | Food-grade polypropylene | Enamelled iron | Glass |
| Vessel shape | Rectangular parallelepiped | Cylinder | Tapered sphere |
| Geometric dimensions, cm | Length 9.0 Width 6.4 Height 4.8 | Radius 20.5 Height 35 | Radius 17.4 Neck radius 10.4 |
| Ratio of growth medium area to vessel neck area | 1:1 | 1:1 | 2.8:1 |
| Growth medium surface area S, cm2 | 65 | 1320 | 946 |
| Scale-up ratio by area | - | 1:20 | 1:15 |
| Vessel volume, L | 0.25 | 45.0 | 17.3 |
| Growth medium volume, L | 0.2 | 8.0 | 8.0 |
| Scale-up ratio by volume | - | 1:40 | 1:40 |
| Growth medium layer height h, cm | 3.6 | 6.1 | from 0 to 12.5 |
| Ratio of S/h, cm | 18 | 218 | from 0 to 76 |
| Gel-Film Removals | I | II | III | IV | V | VI | VII | VIII | IX | X | XI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biosynthesis in small vessel 1 | |||||||||||
| Biosynthesis time for a given BC sample (days) | 3 | 3 | 3 | 5 | 8 | – | – | – | – | – | – |
| Total biosynthesis time (days) | 3 | 6 | 9 | 14 | 22 | – | – | – | – | – | – |
| Biosynthesis in cylinder-shaped vessel 2 | |||||||||||
| Biosynthesis time for a given BC sample (days) | 3 | 3 | 3 | 3 | 4 | 4 | 5 | – | – | – | – |
| Total biosynthesis time (days) | 3 | 6 | 9 | 12 | 16 | 20 | 25 | – | – | – | – |
| Biosynthesis in tapered sphere-shaped vessel 3 | |||||||||||
| Biosynthesis time for a given BC sample (days) | 3 | 3 | 4 | 4 | 5 | 5 | 6 | 6 | 6 | 7 | 11 |
| Total biosynthesis time (days) | 3 | 6 | 10 | 14 | 19 | 24 | 30 | 36 | 42 | 49 | 60 |
| Vessel | 1 | 2 | 3 |
|---|---|---|---|
| Surface area of growth medium S, cm2 | 65 | 1320 | 946 |
| Number of gel-films | 5 | 7 | 11 |
| Overall surface area of BC samples, cm2 | 325 | 9240 | 10,406 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skiba, E.A.; Shavyrkina, N.A.; Budaeva, V.V.; Sitnikova, A.E.; Korchagina, A.A.; Bychin, N.V.; Gladysheva, E.K.; Pavlov, I.N.; Zharikov, A.N.; Lubyansky, V.G.; et al. Biosynthesis of Bacterial Cellulose by Extended Cultivation with Multiple Removal of BC Pellicles. Polymers 2021, 13, 2118. https://doi.org/10.3390/polym13132118
Skiba EA, Shavyrkina NA, Budaeva VV, Sitnikova AE, Korchagina AA, Bychin NV, Gladysheva EK, Pavlov IN, Zharikov AN, Lubyansky VG, et al. Biosynthesis of Bacterial Cellulose by Extended Cultivation with Multiple Removal of BC Pellicles. Polymers. 2021; 13(13):2118. https://doi.org/10.3390/polym13132118
Chicago/Turabian StyleSkiba, Ekaterina A., Nadezhda A. Shavyrkina, Vera V. Budaeva, Anastasia E. Sitnikova, Anna A. Korchagina, Nikolay V. Bychin, Evgenia K. Gladysheva, Igor N. Pavlov, Andrey N. Zharikov, Vladimir G. Lubyansky, and et al. 2021. "Biosynthesis of Bacterial Cellulose by Extended Cultivation with Multiple Removal of BC Pellicles" Polymers 13, no. 13: 2118. https://doi.org/10.3390/polym13132118