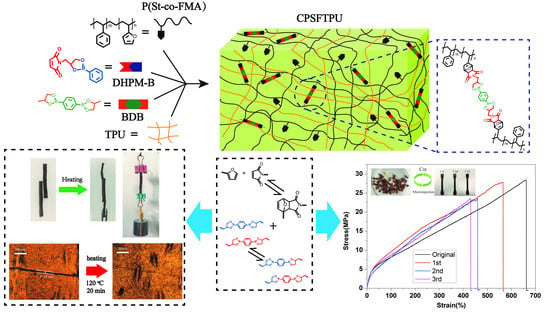
Dynamic Semi IPNs with Duple Dynamic Linkers: Self-Healing, Reprocessing, Welding, and Shape Memory Behaviors
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of 2,2′-(1,4-Phenylene)-bis [4-Methyl-1,3,2-Dioxaborolane] (BDB)
2.3. Synthesis of 1-[(2-Phenyl-1,3,2-Dioxaborolan-4-Yl)Methyl]-1H-Pyrrole-2,5-Dione (DHPM-B)
2.4. Copolymerization of FMA and St (P(St-co-FMA))
2.5. Synthesis of CANs (CPSF CANs)
2.6. Fabrication of Semi-IPN with CPSF and TPU (semi IPNs of CPSFTPU)
2.7. Self-Healing, Shape-Memory, and Reprocessing
2.8. Characterizations
3. Results and Discussions
3.1. Synthesis and Characterization of Semi IPNs CPSFTPU
3.1.1. Characterization of Copolymers P(St-co-FMA)
3.1.2. Characterization of Semi IPNs CPSFTPU
3.2. Dynamic Properties Analysis
3.3. Mechanical and Thermal Properties
3.4. Self-Healing, Welding, and Shape Memory
3.5. Reprocessing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Raquez, J.-M.; Deléglise, M.; Lacrampe, M.-F.; Krawczak, P. Thermosetting (bio)materials derived from renewable resources: A critical review. Prog. Polym. Sci. 2010, 35, 487–509. [Google Scholar] [CrossRef]
- Rutz, B.H.; Berg, J.C. A review of the feasibility of lightening structural polymeric composites with voids without compromising mechanical properties. Adv. Colloid Interface Sci. 2010, 160, 56–75. [Google Scholar] [CrossRef]
- Winne, J.M.; Leibler, L.; Du Prez, F.E. Dynamic covalent chemistry in polymer networks: A mechanistic perspective. Polym. Chem. 2019, 10, 6091–6108. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Bowman, C.N. Covalent adaptable networks: Smart, reconfigurable and responsive network systems. Chem. Soc. Rev. 2013, 42, 7161–7173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.M.; Yu, X.W.; Pei, Z.Q.; Yang, Y.; Wei, Y.; Ji, Y. Multi-stimuli responsive and multi-functional oligoaniline-modified vitrimers. Chem. Sci. 2016, 8, 724–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, F.; Smulders, M.M.J. Dynamic covalent polymers. J. Polym. Sci. Part A: Polym. Chem. 2016, 54, 3551–3577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, W.; Dong, J.; Luo, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: From Old Chemistry to Modern Day Innovations. Adv. Mater. 2017, 29, 1606100. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Scott, T.F.; Adzima, B.J.; Bowman, C.N. Covalent Adaptable Networks (CANs): A Unique Paradigm in Cross-Linked Polymers. Macromolecules 2010, 43, 2643–2653. [Google Scholar] [CrossRef] [Green Version]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-Like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-Catalyzed Transesterification for Healing and Assembling of Thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. [Google Scholar] [CrossRef]
- Stefani, H.A.; Costa, I.M.; Silva, D.D. An easy synthesis of enaminones in water as solvent. Synthesis 2000, 11, 1526–1528. [Google Scholar] [CrossRef]
- Obadia, M.M.; Mudraboyina, B.P.; Serghei, A.; Montarnal, D.; Drockenmuller, E. Reprocessing and Recycling of Highly Cross-Linked Ion-Conducting Networks through Transalkylation Exchanges of C–N Bonds. J. Am. Chem. Soc. 2015, 137, 6078–6083. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.W.; McCarthy, T.J. A Surprise from 1954: Siloxane Equilibration Is a Simple, Robust, and Obvious Polymer Self-Healing Mechanism. J. Am. Chem. Soc. 2012, 134, 2024–2027. [Google Scholar] [CrossRef] [PubMed]
- Vougioukalakis, G.C.; Grubbs, R.H. Ruthenium-Based Heterocyclic Carbene-Coordinated Olefin Metathesis Catalysts. Chem. Rev. 2010, 110, 1746–1787. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Rekondo, A.; De Luzuriaga, A.R.; Cabañero, G.; Grande, H.J.; Odriozola, I. The processability of a poly(urea-urethane) elastomer reversibly crosslinked with aromatic disulfide bridges. J. Mater. Chem. A 2014, 2, 5710–5715. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, Y.L.; Zhang, X.Y.; Tao, L.; Li, S.X.; Wei, Y. Facilely prepared inexpensive and biocompatible self-healing hydrogel: A new injectable cell therapy carrier. Polym. Chem. 2012, 3, 3235–3238. [Google Scholar] [CrossRef]
- Cromwell, O.R.; Chung, J.; Guan, Z.B. Malleable and Self-Healing Covalent Polymer Networks through Tunable Dynamic Boronic Ester Bonds. J. Am. Chem. Soc. 2015, 137, 6492–6495. [Google Scholar] [CrossRef]
- Nishimura, Y.; Chung, J.; Muradyan, H.; Guan, Z.B. Silyl Ether as a Robust and Thermally Stable Dynamic Covalent Motif for Malleable Polymer Design. J. Am. Chem. Soc. 2017, 139, 14881–14884. [Google Scholar] [CrossRef]
- Zhang, X.N.; Wang, Y.J.; Sun, S.T.; Hou, L.; Wu, P.; Wu, Z.L.; Zheng, Q. A Tough and Stiff Hydrogel with Tunable Water Content and Mechanical Properties Based on the Synergistic Effect of Hydrogen Bonding and Hydrophobic Interaction. Macromolecules 2018, 51, 8136–8146. [Google Scholar] [CrossRef]
- Tang, Z.H.; Huang, J.; Guo, B.C.; Zhang, L.Q.; Liu, F. Bioinspired Engineering of Sacrificial Metal–Ligand Bonds into Elastomers with Supramechanical Performance and Adaptive Recovery. Macromolecules 2016, 49, 1781–1789. [Google Scholar] [CrossRef]
- Ducrot, E.; Chen, Y.; Bulters, M.; Sijbesma, R.P.; Creton, C. Toughening Elastomers with Sacrificial Bonds and Watching Them Break. Science 2014, 344, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Kalogeras, I.M.; Stathopoulos, A.; Vassilikou-Dova, A.; Brostow, W. Nanoscale Confinement Effects on the Relaxation Dynamics in Networks of Diglycidyl Ether of Bisphenol-A and Low-Molecular-Weight Poly(ethylene oxide). J. Phys. Chem. B 2007, 111, 2774–2782. [Google Scholar] [CrossRef] [PubMed]
- Kayaman, N.; Hamurcu, E.E.G.; Uyanik, N.; Baysal, B.M. Interpenetrating hydrogel networks based on polyacrylamide and poly(itaconic acid): Synthesis and characterization. Macromol. Chem. Phys. 1999, 200, 231–238. [Google Scholar] [CrossRef]
- Liu, G.Q.; Guan, C.L.; Xia, H.S.; Guo, F.Q.; Ding, X.B.; Peng, Y.X. Novel Shape-Memory Polymer Based on Hydrogen Bonding. Macromol. Rapid Commun. 2006, 27, 1100–1104. [Google Scholar] [CrossRef]
- James, J.; Thomas, G.V.; Pramoda, K.; Thomas, S. Transport behaviour of aromatic solvents through styrene butadiene rubber/poly [methyl methacrylate] (SBR/PMMMA) interpenetrating polymer network (IPN) membranes. Polymer 2017, 116, 76–88. [Google Scholar] [CrossRef]
- Jeon, O.; Shin, J.-Y.; Marks, R.; Hopkins, M.; Kim, T.-H.; Park, H.-H.; Alsberg, E. Highly Elastic and Tough Interpenetrating Polymer Network-Structured Hybrid Hydrogels for Cyclic Mechanical Loading-Enhanced Tissue Engineering. Chem. Mater. 2017, 29, 8425–8432. [Google Scholar] [CrossRef]
- Ube, T.; Minagawa, K.; Ikeda, T. Interpenetrating polymer networks of liquid-crystalline azobenzene polymers and poly(dimethylsiloxane) as photomobile materials. Soft Matter 2017, 13, 5820–5823. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.M.; Lensmeyer, E.E.; Zhang, B.; Chakma, P.; Flum, J.A.; Via, J.J.; Sparks, J.L.; Konkolewicz, D. Effect of Polymer Network Architecture, Enhancing Soft Materials Using Orthogonal Dynamic Bonds in an Interpenetrating Network. ACS Macro Lett. 2017, 6, 495–499. [Google Scholar] [CrossRef]
- Poomali; Siddaramaiah; Suresha, B.; Lee, J.-H. Mechanical and three-body abrasive wear behaviour of PMMA/TPU blends. Mater. Sci. Eng. A 2008, 492, 486–490. [Google Scholar] [CrossRef]
- Rossa-Sierra, A.; Sanchez-Soto, M.; Illescas, S.; Maspoch, M. Study of the interface behaviour between MABS/TPU bi-layer structures obtained through over moulding. Mater. Des. 2009, 30, 3979–3988. [Google Scholar] [CrossRef]
- Simmons, A.; Hyvarinen, J.; Poole-Warren, L. The effect of sterilisation on a poly(dimethylsiloxane)/poly(hexamethylene oxide) mixed macrodiol-based polyurethane elastomer. Biomaterials 2006, 27, 4484–4497. [Google Scholar] [CrossRef] [PubMed]
- Vakil, J.R.; De Alwis Watuthanthrige, N.; Digby, Z.A.; Zhang, B.; Lacy, H.A.; Sparks, J.L.; Konkolewicz, D. Controlling polymer architecture to design dynamic network materials with multiple dynamic linkers. Mol. Syst. Des. Eng. 2020, 5, 1267–1276. [Google Scholar] [CrossRef]
- Wei, H.-L.; Yang, Z.; Zheng, L.-M.; Shen, Y.-M. Thermosensitive hydrogels synthesized by fast Diels–Alder reaction in water. Polymer 2009, 50, 2836–2840. [Google Scholar] [CrossRef]
- Röttger, M.; Domenech, T.; Van Der Weegen, R.; Nicolaÿ, R.; Leibler, L. High-performance vitrimers from commodity thermoplastics through dioxaborolane metathesis. Science 2017, 356, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, R.; Wu, Q.; Chen, T.; Sun, P. Bio-Inspired High-Performance and Recyclable Cross-Linked Polymers. Adv. Mater. 2013, 25, 4912–4917. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.; Park, J.S.; Shin, C.H.; Choi, J.W.; Chun, B.C. Grafting of shape memory polyurethane with poly(ethyleneimine) and the effect on electrolytic attraction in aqueous solution and shape recovery properties. Macromol. Res. 2011, 20, 66–75. [Google Scholar] [CrossRef]
- Garg, H.; Mohanty, J.; Gupta, P.; Das, A.; Tripathi, B.P.; Kumar, B. Polyethylenimine-Based Shape Memory Polyurethane with Low Transition Temperature and Excellent Memory Performance. Macromol. Mater. Eng. 2020, 305, 2000215. [Google Scholar] [CrossRef]
- Körpınar, B.; Öztürk, B.C.; Çam, N.F.; Akat, H. Investigations on thermal and radiation shielding properties of the poly(hydroxyethyl methacrylate-co-styrene)/tungsten(VI) oxide composites. Prog. Nucl. Energy 2020, 126, 103424. [Google Scholar] [CrossRef]
- Kavitha, A.A.; Singha, N.K. Atom-Transfer Radical Copolymerization of Furfuryl Methacrylate (FMA) and Methyl Methacrylate (MMA): A Thermally-Amendable Copolymer. Macromol. Chem. Phys. 2007, 208, 2569–2577. [Google Scholar] [CrossRef]
- Zeng, Y.; Mahmood, Q.; Liang, T.; Sun, W.-H. (Co-)polymerization of methylacrylate with NBE/1-hexene by (8-arylimino-5,6,7-trihydroquinolyl)(methyl)palladium chlorides: An approaching mechanism and the polymeric microstructures. New J. Chem. 2017, 41, 3653–3660. [Google Scholar] [CrossRef]
- Aizpurua, J.; Martin, L.; Fernández, M.; González, A.; Irusta, L. Recyclable, remendable and healing polyurethane/acrylic coatings from UV curable waterborne dispersions containing Diels-Alder moieties. Prog. Org. Coat. 2020, 139, 105460. [Google Scholar] [CrossRef]
- Ji, F.; Li, J.; Zhang, G.; Lan, W.; Sun, R.; Wong, C.-P. Alkaline monomer for mechanical enhanced and self-healing hydrogels based on dynamic borate ester bonds. Polymer 2019, 184, 121882. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Z.H.; Zhang, X.H.; Liu, Y.J.; Wu, S.W.; Guo, B.C. Covalently Cross-Linked Elastomers with Self-Healing and Malleable Abilities Enabled by Boronic Ester Bonds. ACS Appl. Mater. Interfaces 2018, 10, 24224–24231. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.; Ci, M.; Sui, S.; Zhu, P. Study on the rheological properties of regenerated cellulose/thermoplastic polyurethane blend spinning solutions. Ferroelectrics 2020, 562, 104–113. [Google Scholar] [CrossRef]
- Sigalov, M.V.; Shainyan, B.A.; Chipanina, N.N.; Oznobikhina, L.P.; Kuzmin, A.V. E→Z photoinduced isomerization and hydrogen bonding in the peri-acetamido substituted (1H-pyrrol-2-ylmethylene)benzocycloalkanones. Tetrahedron 2020, 76, 131202. [Google Scholar] [CrossRef]
- Suo, X.; Cao, Z.; Yu, Y.; Liu, Y. Dynamic self-stiffening in polyacrylonitrile/thermoplastic polyurethane composites. Compos. Sci. Technol. 2020, 198, 108256. [Google Scholar] [CrossRef]
- Qu, Z.; Mi, J.; Jiao, Y.; Zhou, H.; Wang, X. Microcellular morphology evolution of polystyrene/thermoplastic polyurethane blends in the presence of supercritical CO2. Cell. Polym. 2019, 38, 68–85. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Li, H.; Zhou, H. Thermoplastic polyurethane ( TPU ) modifier to develop bimodal cell structure in polypropylene/ TPU microcellular foam in presence of supercritical CO 2. J. Vinyl Addit. Technol. 2021, 27, 127–136. [Google Scholar] [CrossRef]
- Zhao, Q.; Zou, W.K.; Luo, Y.W.; Xie, T. Shape memory polymer network with thermally distinct elasticity and plasticity. Sci. Adv. 2016, 2, 1501297. [Google Scholar] [CrossRef] [Green Version]
- Capelot, M.; Unterlass, M.M.; Tournilhac, F.; Leibler, L. Catalytic Control of the Vitrimer Glass Transition. ACS Macro Lett. 2012, 1, 789–792. [Google Scholar] [CrossRef]
- Brutman, J.P.; Delgado, P.A.; Hillmyer, M.A. Polylactide Vitrimers. ACS Macro Lett. 2014, 3, 607–610. [Google Scholar] [CrossRef] [Green Version]
- Kuang, X.; Liu, G.; Dong, X.; Wang, D. Correlation between stress relaxation dynamics and thermochemistry for covalent adaptive networks polymers. Mater. Chem. Front. 2017, 2017 1, 111–118. [Google Scholar] [CrossRef]
- Sheridan, R.; Bowman, C.N. A Simple Relationship Relating Linear Viscoelastic Properties and Chemical Structure in a Model Diels–Alder Polymer Network. Macromolecules 2012, 45, 7634–7641. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Z.H.; Liu, Y.J.; Wu, S.W.; Guo, B.C. Mechanically Robust, Self-Healable, and Reprocessable Elastomers Enabled by Dynamic Dual Cross-Links. Macromolecules 2019, 52, 3805–3812. [Google Scholar] [CrossRef]
- Elling, B.R.; Dichtel, W.R. Reprocessable Cross-Linked Polymer Networks: Are Associative Exchange Mechanisms Desirable? ACS Cent. Sci. 2020, 6, 1488–1496. [Google Scholar] [CrossRef]
- Yang, F.; Pan, L.; Ma, Z.; Lou, Y.; Li, Y.; Li, Y. Highly elastic, strong, and reprocessable cross-linked polyolefin elastomers enabled by boronic ester bonds. Polym. Chem. 2020, 11, 3285–3295. [Google Scholar] [CrossRef]
- Liu, H.C.; Zhang, H.; Wang, H.; Huang, X.; Huang, G.S.; Wu, J.R. Weldable, malleable and programmable epoxy vitrimers with high mechanical properties and water insensitivity. Chem. Eng. J. 2019, 368, 61–70. [Google Scholar] [CrossRef]
- Liu, T.; Hao, C.; Wang, L.W.; Li, Y.Z.; Liu, W.C.; Xin, J.N.; Zhang, J.W. Eugenol-Derived Biobased Epoxy: Shape Memory, Repairing, and Recyclability. Macromolecules 2017, 50, 8588–8597. [Google Scholar] [CrossRef]
- Xu, Y.; Odelius, K.; Hakkarainen, M. Photocurable, Thermally Reprocessable, and Chemically Recyclable Vanillin-Based Imine Thermosets. ACS Sustain. Chem. Eng. 2020, 8, 17272–17279. [Google Scholar] [CrossRef]
- Ogden, W.A.; Guan, Z.B. Recyclable, Strong, and Highly Malleable Thermosets Based on Boroxine Networks. J. Am. Chem. Soc. 2018, 140, 6217–6220. [Google Scholar] [CrossRef]














| Samples with Different Monomer Feeding Ratio (St/FMA) | Integration of Proton on –CH2– | Integration of Proton on Benzene |
|---|---|---|
| 8:2 | 6.3 | 591.3 |
| 6:4 | 2.1 | 59.3 |
| 4:6 | 1.9 | 29.1 |
| 2:8 | 11.2 | 36.6 |
| Sample | Breaking Strain (%) | Tensile Stress (MPa) | Young’s Modulus (MPa) |
|---|---|---|---|
| TPU | 651.4 ± 10.11 | 9.7 ± 1.11 | 11.1 ± 1.35 |
| 6%CPSFTPU0.5 | 485.7 ± 12.18 | 12.3 ± 2.18 | 52.8 ± 5.39 |
| 6%CPSFTPU1 | 661.7 ± 8.37 | 28.4 ± 1.37 | 30.2 ± 3.38 |
| 6%CPSFTPU2 | 459.9 ± 10.52 | 27.3 ± 2.52 | 29.4 ± 3.25 |
| Sample | Breaking Strain (%) | Tensile Stress (MPa) | Young’s Modulus (MPa) |
|---|---|---|---|
| 0%CPSFTPU1 | 550.1 ± 12.18 | 7.5 ± 2.18 | 24.5 ± 5.39 |
| 3%CPSFTPU1 | 412.8 ± 10.52 | 19.3 ± 2.52 | 23.5 ± 3.25 |
| 6%CPSFTPU1 | 661.7 ± 8.37 | 28.4 ± 1.37 | 30.2 ± 3.38 |
| 9%CPSFTPU2 | 621.2 ± 12.36 | 29.0 ± 2.77 | 20.1 ± 2.79 |
| Sample | T5d (°C) | T30d (°C) | Residual Weight at 500 °C (%) | Residual Weight at 800 °C (%) |
|---|---|---|---|---|
| TPU | 295.3 | 350.5 | 5.5 | 3.6 |
| BDB | 141.3 | 179.7 | 0.0 | 0.0 |
| DHPM-B | 166.8 | 205.7 | 4.2 | 3.5 |
| P(St-co-FMA) | 225.7 | 369.7 | 5.8 | 3.3 |
| 0%CPSFTPU1 | 280.1 | 356.1 | 12.9 | 9.6 |
| 6%CPSFTPU1 | 305.5 | 356.8 | 17.7 | 14.5 |
| Generation | Breaking Strain Recovery Ratio (%) | Tensile Stress Recovery Ratio (%) | Young’s Modulus Recovery Ratio (%) |
|---|---|---|---|
| 1st | 85.4 ± 5.27 | 95.4 ± 7.52 | 110.9 ± 9.36 |
| 2nd | 69.7 ± 4.81 | 82.2 ± 5.37 | 120.0 ± 10.74 |
| 3rd | 65.0 ± 5.11 | 82.2 ± 3.02 | 79.1 ± 6.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Yang, W.; Liu, S.; Shi, X.; Xi, A.; Zhang, F. Dynamic Semi IPNs with Duple Dynamic Linkers: Self-Healing, Reprocessing, Welding, and Shape Memory Behaviors. Polymers 2021, 13, 1679. https://doi.org/10.3390/polym13111679
Zeng Y, Yang W, Liu S, Shi X, Xi A, Zhang F. Dynamic Semi IPNs with Duple Dynamic Linkers: Self-Healing, Reprocessing, Welding, and Shape Memory Behaviors. Polymers. 2021; 13(11):1679. https://doi.org/10.3390/polym13111679
Chicago/Turabian StyleZeng, Yanning, Weiming Yang, Shuxin Liu, Xiahui Shi, Aoqian Xi, and Faai Zhang. 2021. "Dynamic Semi IPNs with Duple Dynamic Linkers: Self-Healing, Reprocessing, Welding, and Shape Memory Behaviors" Polymers 13, no. 11: 1679. https://doi.org/10.3390/polym13111679