Influence of the Glass Transition Temperature and the Density of Crosslinking Groups on the Reversibility of Diels-Alder Polymer Networks
Abstract
:1. Introduction
- (i)
- Polymers can be either crosslinked via non-covalent interactions following the concepts of “supramolecular chemistry” [6,7]. Supramolecular polymer networks building blocks are held together by relatively weak intermolecular or intramolecular interactions, such as hydrogen bonds [8,9,10,11,12], π-π interactions [13,14], or metal-ligand interactions [15,16,17]. An advantage of these types of interactions is that they open and close fast upon increasing and lowering temperature. In principle, thermoplastics and thermoplastic elastomers based on semicrystalline polymers or hard-soft segmented and microphase separated block copolymers with at least two hard blocks can be considered as thermo-reversible networks, where processability occurs above the melting point or the order-disorder transition. However, these systems typically still have a high melt viscosity because the molecular weights are high. However, low viscosity of the polymer melt can be achieved if low molecular weight, unentangled polymers can be used, which facilitates the production and processing of products.
- (ii)
- Polymers can also be covalently, reversibly crosslinked leading to “covalent adaptable networks” (CANs) [18]. Dissociative and associative CANs can be distinguished. This subdivision is based on the type of exchange reaction of the crosslinks. If the network is broken during the reaction, it is called a dissociative CAN (Figure 1a), like in the case of the Diels-Alder reaction. If the network is only changed during an exchange reaction but remains permanently crosslinked, it is called an associative CAN (Figure 1b) [19]. Vitrimers are typical examples for associative CANs [19].
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Furan Protected Maleimide Methacrylate (MIMA)
2.3. Synthesis of Furfuryl Bearing Polymers by ATRP
2.4. Synthesis of Maleimide Bearing Copolymer by Free Radical Polymerization
2.5. Crosslinking of Furfuryl Bearing Polymers
2.5.1. Crosslinking at Room Temperature
2.5.2. Crosslinking at 120 °C
2.5.3. Crosslinking at 120 °C and 165 °C
2.6. Characterization
2.6.1. Size Exclusion Chromatography
2.6.2. Nuclear Magnetic Resonance Spectroscopy
2.6.3. Fourier Transform Infrared Spectroscopy
2.6.4. Differential Scanning Calorimetry
3. Results and Discussion
3.1. Polymer Synthesis
3.2. Crosslinking of Furfuryl Bearing Polymers
3.2.1. Visual Observation of Gelation
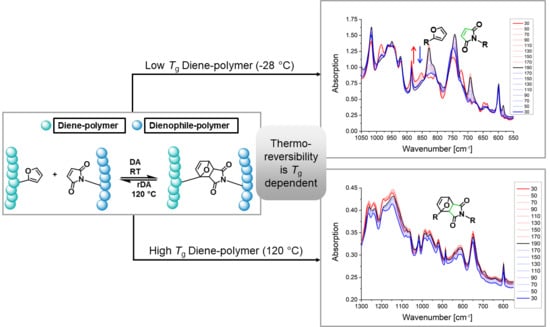
3.2.2. Thermo-Reversibility of Gelation
3.2.3. Self-Healing Ability of Crosslinked Furfuryl Bearing Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dübner, M.; Spencer, N.D.; Padeste, C. Light-responsive polymer surfaces via postpolymerization modification of grafted polymer-brush structures. Langmuir 2014, 30, 14971–14981. [Google Scholar] [CrossRef]
- Zhang, Q.; Xia, F.; Sun, T.; Song, W.; Zhao, T.; Liu, M.; Jiang, L. Wettability switching between high hydrophilicity at low pH and high hydrophobicity at high pH on surface based on pH-responsive polymer. Chem. Commun. 2008, 10, 1199–1201. [Google Scholar] [CrossRef]
- Kim, P.; Hu, Y.; Alvarenga, J.; Kolle, M.; Suo, Z.; Aizenberg, J. Rational Design of Mechano-Responsive Optical Materials by Fine Tuning the Evolution of Strain-Dependent Wrinkling Patterns. Adv. Opt. Mater. 2013, 1, 381–388. [Google Scholar] [CrossRef]
- Ichimura, K.; Oh, S.K.; Nakagawa, M. Light-driven motion of liquids on a photoresponsive surface. Science 2000, 288, 1624–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host guest polymers. Nat. Commun. 2011, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- De Greef, T.F.A.; Meijer, E.W. Supramolecular Polymers. Nature 2008, 453, 171–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehn, J.M. Towards complex matter: Supramolecular chemistry and self-organization. Eur. Rev. 2009, 17, 263–280. [Google Scholar] [CrossRef] [Green Version]
- Sijbesma, R.P.; Beijer, F.H.; Brunsveld, L.; Folmer, B.J.B.; Hirschberg, J.H.K.K.; Lange, R.F.M.; Lowe, J.K.L.; Meijer, E.W. Reversible polymers formed from self-complementary monomers using quadruple hydrogen bonding. Science 1997, 278, 1601–1604. [Google Scholar] [CrossRef]
- Mes, T.; Koenigs, M.M.E.; Scalfani, V.F.; Bailey, T.S.; Meijer, E.W.; Palmans, A.R.A. Network formation in an orthogonally self-assembling system. ACS Macro Lett. 2012, 1, 105–109. [Google Scholar] [CrossRef]
- Rahmstorf, E.; Abetz, V. Supramolecular networks from block copolymers based on styrene and isoprene using hydrogen bonding motifs-Part 2: Dynamic mechanical analysis. Materials 2018, 11, 1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittenberg, E.; Abetz, V. New post modification route for styrene butadiene copolymers leading to supramolecular hydrogen bonded networks—Synthesis and thermodynamic analysis of complexation. Polymer 2017, 121, 304–311. [Google Scholar] [CrossRef]
- Hilger, C.; Stadler, R. Cooperative structure formation by combination of covalent and association chain polymers: 4. Designing functional groups for supramolecular structure formation. Polymer 1991, 32, 3244–3249. [Google Scholar] [CrossRef]
- Burattini, S.; Greenland, B.W.; Merino, D.H.; Weng, W.; Seppala, J.; Colquhoun, H.M.; Hayes, W.; MacKay, M.E.; Hamley, I.W.; Rowan, S.J. A healable supramolecular polymer blend based on aromatic π-π Stacking and hydrogen-bonding interactions. J. Am. Chem. Soc. 2010, 132, 12051–12058. [Google Scholar] [CrossRef] [PubMed]
- Burattini, S.; Greenland, B.W.; Hayes, W.; MacKay, M.E.; Rowan, S.J.; Colquhoun, H.M. A supramolecular polymer based on tweezer-type π-π stacking interactions: Molecular design for healability and enhanced toughness. Chem. Mater. 2011, 23, 6–8. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Takashima, Y.; Hashidzume, A.; Yamaguchi, H.; Harada, A. Reversible self-assembly of gels through metal-ligand interactions. Sci. Rep. 2013, 3, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Dobrawa, R.; Würthner, F. Metallosupramolecular approach toward functional coordination polymers. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4981–4995. [Google Scholar] [CrossRef]
- Hofmeier, H.; Schubert, U.S. Recent developments in the supramolecular chemistry of terpyridine-metal complexes. Chem. Soc. Rev. 2004, 33, 373–399. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Scott, T.F.; Adzima, B.J.; Bowman, C.N. Covalent adaptable networks (CANs): A unique paradigm in cross-linked polymers. Macromolecules 2010, 43, 2643–2653. [Google Scholar] [CrossRef] [Green Version]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Hizal, G.; Tunca, U.; Sanyal, A. Discrete macromolecular constructs via the Diels-Alder “click” reaction. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4103–4120. [Google Scholar] [CrossRef]
- Munirasu, S.; Albuerne, J.; Boschetti-de-Fierro, A.; Abetz, V. Functionalization of Carbon Materials using the Diels-Alder Reaction. Macromol. Rapid Commun. 2010, 31, 574–579. [Google Scholar] [CrossRef] [Green Version]
- Kuhrau, M.; Stadler, R. Synthesis of new polymers via Diels-Alder reaction. Die Makromol. Chem. Rapid Commun. 1990, 11, 635–644. [Google Scholar] [CrossRef]
- Kuhrau, M.; Stadler, R. Synthesis of new polymers via Diels-Alder reaction, 2. Soluble polymers via copolymerization. Die Makromol. Chem. 1992, 193, 2861–2874. [Google Scholar] [CrossRef]
- Mallakpour, S.E.; Kolshorn, H.; Schollmeyer, D.; Stadler, R. Step growth polymerization via tandem ene and Diels-Alder reactions. Macromol. Chem. Phys. 1997, 198, 251–263. [Google Scholar] [CrossRef]
- Tasdelen, M.A. Diels–Alder “click” reactions: Recent applications in polymer and material science. Polym. Chem. 2011, 2, 2133. [Google Scholar] [CrossRef]
- Canary, S.A.; Stevens, M.P. Thermally reversible crosslinking of polystyrene via the furan–maleimide Diels–Alder reaction. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 1755–1760. [Google Scholar] [CrossRef]
- Goussé, C.; Gandini, A.; Hodge, P. Application of the Diels−Alder Reaction to Polymers Bearing Furan Moieties. 2. Diels−Alder and Retro-Diels−Alder Reactions Involving Furan Rings in Some Styrene Copolymers. Macromolecules 1998, 31, 314–321. [Google Scholar] [CrossRef]
- Toncelli, C.; De Reus, D.C.; Picchioni, F.; Broekhuis, A.A. Properties of Reversible Diels—Alder Furan/Maleimide Polymer Networks as Function of Crosslink Density. Macromol. Chem. Phys. 2012, 213, 157–165. [Google Scholar] [CrossRef]
- Zhang, Y.; Broekhuis, A.A.; Picchioni, F. Thermally Self-Healing Polymeric Materials: The Next Step to Recycling Thermoset Polymers? Macromolecules 2009, 42, 1906–1912. [Google Scholar] [CrossRef] [Green Version]
- Kavitha, A.A.; Singha, N.K. Atom-transfer radical copolymerization of furfuryl methacrylate (FMA) and methyl methacrylate (MMA): A thermally-amendable copolymer. Macromol. Chem. Phys. 2007, 208, 2569–2577. [Google Scholar] [CrossRef]
- Kavitha, A.A.; Singha, N.K. Atom transfer radical polymerization (ATRP) of methyl methacrylate using a functional initiator bearing an amino-adamantane. Macromol. Chem. Phys. 2009, 210, 1536–1543. [Google Scholar] [CrossRef]
- Polgar, L.M.; Van Duin, M.; Broekhuis, A.A.; Picchioni, F. Use of Diels-Alder Chemistry for Thermoreversible Cross-Linking of Rubbers: The Next Step toward Recycling of Rubber Products? Macromolecules 2015, 48, 7096–7105. [Google Scholar] [CrossRef]
- Moustafa, M.M.A.R.; Gillies, E.R. Rubber functionalization by diels-alder chemistry: From cross-linking to multifunctional graft copolymer synthesis. Macromolecules 2013, 46, 6024–6030. [Google Scholar] [CrossRef]
- Polgar, L.M.; Kingma, A.; Roelfs, M.; van Essen, M.; van Duin, M.; Picchioni, F. Kinetics of cross-linking and de-cross-linking of EPM rubber with thermoreversible Diels-Alder chemistry. Eur. Polym. J. 2017, 90, 150–161. [Google Scholar] [CrossRef]
- Tanasi, P.; Santana, M.H.; Carretero-González, J.; Verdejo, R.; López-Manchado, M.A. Thermo-reversible crosslinked natural rubber: A Diels-Alder route for reuse and self-healing properties in elastomers. Polymer 2019, 175, 15–24. [Google Scholar] [CrossRef]
- Sanyal, A. Diels-alder cycloaddition-cycloreversion: A powerful combo in materials design. Macromol. Chem. Phys. 2010, 211, 1417–1425. [Google Scholar] [CrossRef]
- Engel, T.; Kickelbick, G. Self-healing nanocomposites from silica-polymer core-shell nanoparticles. Polym. Int. 2014, 63, 915–923. [Google Scholar] [CrossRef]
- Schäfer, S.; Kickelbick, G. Self-healing polymer nanocomposites based on Diels-Alder-reactions with silica nanoparticles: The role of the polymer matrix. Polymer 2015, 69, 357–368. [Google Scholar] [CrossRef]
- Zeng, C.; Seino, H.; Ren, J.; Hatanaka, K.; Yoshie, N. Self-healing bio-based furan polymers cross-linked with various bis-maleimides. Polymer 2013, 54, 5351–5357. [Google Scholar] [CrossRef]
- Klein, R.; Übel, F.; Frey, H. Maleimide Glycidyl Ether: A Bifunctional Monomer for Orthogonal Cationic and Radical Polymerizations. Macromol. Rapid Commun. 2015, 36, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, A.; Göstl, R.; Wendt, R.; Kötteritzsch, J.; Hager, M.D.; Schubert, U.S.; Brademann-Jock, K.; Thünemann, A.F.; Nöchel, U.; Behl, M.; et al. Conditional repair by locally switching the thermal healing capability of dynamic covalent polymers with light. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dispinar, T.; Sanyal, R.; Sanyal, A. A Diels-Alder/Retro Diels-Alder Strategy to Synthesize Polymers Bearing Maleimide Side Chains. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 4545–4551. [Google Scholar] [CrossRef]
- Arunbabu, D.; Noh, S.M.; Nam, J.H.; Oh, J.K. Thermoreversible Self-Healing Networks Based on a Tunable Polymethacrylate Crossslinker Having Pendant Maleimide Groups. Macromol. Chem. Phys. 2016, 217, 2191–2198. [Google Scholar] [CrossRef]














| Polymer | Mn [kDa] | Mw [kDa] | Ð | Df [wt%] | Tg [°C] |
|---|---|---|---|---|---|
| PFMA5 | 4.7 | 6.6 | 1.4 | 100 | 39 |
| P(MMA89-co-FMA11)24 | 24.0 | 40.0 | 1.7 | 11 | 109 |
| P(BA60-co-FMA40)10 | 10.4 | 18.1 | 1.7 | 40 | −17 |
| P(MMA74-co-MIMA26)9 | 9.0 | 16.2 | 1.8 | 26 | 135 |
| Crosslinking Components | PFMA5 + BMI | PFMA5 + P(MMA74-co-MIMA26)24 | ||||||
| Motif Ratio | 1:2 | 1:1 | 1:1 | |||||
| Reaction Temperature [°C] | 25 | 120, 25 | 25 | 120, 25 | 25 | 120, 165 | ||
| Reaction time [h] | 72 | 2, 12 | 72 | 2, 12 | 168 | 12, 5 | ||
| solvent | DCM | DCM | DCM | DCM | DCM | anisole | ||
| gelation | + | + | + | + | + | + | ||
| color | yellow | caramel, turbid | yellow | caramel, turbid | light yellow | black/dark brown | ||
| Crosslinking Components | P(MMA89-co-FMA11)9 + BMI | P(MMA89-co-FMA11)9 + P(MMA74-co-MIMA26)24 | ||||||
| Motif Ratio | 1:2 | 1:1 | 1:0.5 | 1:1 | ||||
| Reaction Temperature [°C] | 25 | 120, 25 | 25 | 120, 25 | 25 | 120, 25 | 25 | 120, 165 |
| Reaction time [h] | 72 | 2, 12 | 72 | 2, 12 | 72 | 2, 12 | 72 | 12, 5 |
| solvent | DCM | DCM | DCM | DCM | DCM | DCM | DCM | anisole |
| gelation | + * | + | + * | + | + * | + | + | + * |
| color | light yellow | yellow | light yellow | yellow | light yellow | yellow | light yellow, turbid | yellow |
| Crosslinking Components | P(BA60-co-FMA40)10 + BMI | P(BA60-co-FMA40)10 + P(MMA74-co-MIMA26)24 | ||||||
| Motif Ratio | 1:2 | 1:1 | 1:0.5 | 1:1 | ||||
| Reaction Temperature [°C] | 25 | 120, 25 | 25 | 120, 25 | 25 | 120, 25 | 25 | 120, 165 |
| Reaction time [h] | 72 | 2, 12 | 72 | 2, 12 | 72 | 2, 12 | 168 | 12, 5 |
| solvent | DCM | DCM | DCM | DCM | DCM | DCM | DCM | anisole |
| gelation | + * | + * | + * | + * | + * | + * | + | + * |
| color | light yellow | light yellow | light yellow | light yellow | light yellow | light yellow | light yellow | dark yellow |
| Polymer with Crosslinking Agent and Conditions | rDA3/rDA2 |
|---|---|
| PFMA5 with BMI; 1:1 at rt | 0.90 |
| PFMA5 with BMI; 1:2 at rt | 0.91 |
| PFMA5 with BMI; 1:1 at 120 and 165 °C | 1.0 |
| PFMA5 with BMI; 1:2 at 120 and 165 °C | 1.0 |
| PFMA5 with P(MMA74-co-MIMA26)9; 1:1 at rt | 0.89 |
| P(MMA89-co-FMA11)24 with BMI; 1:1 at rt | 0 |
| P(MMA89-co-FMA11)24 with BMI; 1:1 at 120 °C | 0 |
| P(MMA89-co-FMA11)24 with BMI; 1:1 at rt; 5 wt% anisole | 0 |
| P(MMA89-co-FMA11)24 with BMI; 1:1 at rt; 10 wt% anisole | 0.84 |
| P(MMA89-co-FMA11)24 with BMI; 1:1 at rt; 25 wt% anisole | 0.60 |
| P(MMA89-co-FMA11)24 with BMI; 1:1 at rt; 50 wt% anisole | 0 |
| P(MMA89-co-FMA11)24 with P(MMA74-co-MIMA26)9; 1:1 at rt | 0 |
| P(BA60-co-FMA40)10 with BMI; 1:1 at rt | 1.0 |
| P(BA60-co-FMA40)10 with BMI; 1:2 at rt | 0.98 |
| P(BA60-co-FMA40)10 with P(MMA74-co-MIMA26)9; 1:1 at rt | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiessen, M.; Abetz, V. Influence of the Glass Transition Temperature and the Density of Crosslinking Groups on the Reversibility of Diels-Alder Polymer Networks. Polymers 2021, 13, 1189. https://doi.org/10.3390/polym13081189
Thiessen M, Abetz V. Influence of the Glass Transition Temperature and the Density of Crosslinking Groups on the Reversibility of Diels-Alder Polymer Networks. Polymers. 2021; 13(8):1189. https://doi.org/10.3390/polym13081189
Chicago/Turabian StyleThiessen, Merlina, and Volker Abetz. 2021. "Influence of the Glass Transition Temperature and the Density of Crosslinking Groups on the Reversibility of Diels-Alder Polymer Networks" Polymers 13, no. 8: 1189. https://doi.org/10.3390/polym13081189