Polymeric Dopant-Free Hole Transporting Materials for Perovskite Solar Cells: Structures and Concepts towards Better Performances
Abstract
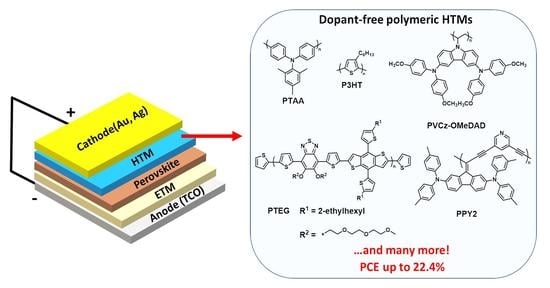
:1. Introduction
2. Perovskite Solar Cells: The Essential
2.1. Perovskite Structure and Properties
2.2. Deposition Method of Perovskite for PSCs
2.3. Perovskite Solar Cells—PSCs
3. Organic HTMs
3.1. Organic HTMs, Doping and Dopant-Free HTMs
3.2. Dopant-Free Organic HTM Polymers—Why?
3.3. Organic HTM Polymer Classification
3.3.1. Homopolymers
3.3.2. D-A Copolymers
3.3.2.1. DPP (2,5-Dihydropyrrolo[3,4]Pyrrole-1,4-Dione)
3.3.2.2. BDT (Benzo[1,2-b:4,5-b’]Dithiophene)
3.3.2.3. BTZ (Benzothiadiazole) and Cbz (Carbazole)
3.3.2.4. Other Polymers
4. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AgTFSI | Silver bis(TriFluoromethane-Sulfonyl)Imide |
| ALD-TiO2 | Atomic Layer Deposited Titanium dioxide thin film |
| BCF | Tris(pentaflurophenyl)borane |
| BDT | Benzodithiophene |
| BG or Bg | Band-Gap |
| BIPV | Building-integrated photovoltaics |
| BTZ | Benzothiadiazole |
| CB | Conduction Band |
| CBz | Carbazole |
| CsPbI3 | Cesium Lead Iodide Perovskite |
| CsFAMA | Cesium FormAmidinium/MethylAmmonium Perovskite |
| c-TiO2 | Compact Titanium Dioxide |
| CV | Cyclic Voltammetry |
| D-A | Donor–Acceptor |
| D-π-A | Donor–π-conjugated bridge–Acceptor |
| DFT | Density Functional Theory |
| DSC or DSSCs | Dye-Sensitized Solar Cells |
| ETL | Electron Transporting Layer |
| ETM | Electron Transporting Material |
| FA | Formamidinium |
| FAMA | Formamidinium methylammonium |
| FAPbI3 | FormAmidinium Lead triiodide Perovskite |
| FF | Fill Factor |
| FTO | Fluorine doped Tin Oxide |
| GIWAXS | Grazing Incidence Wide Angle X-Ray Scattering |
| HM | Hole Mobility |
| HOMO | Highest Occupied Molecular Orbital |
| HTL | Hole Transporting Layer |
| HTM | Hole Transporting Material |
| IDT | Indacenothiophene |
| IDTT | Indacenodithieno[3,2-b]thiophene T |
| Jsc | Short Circuit Current Density |
| LiTFSI | Lithium bis(TriFluoromethylSulfonyl)Imide |
| LUMO | Lowest Unoccupied Molecular Orbital |
| Mn | Number Average Molecular Weight |
| Mw | Weight Average Molecular Weight |
| MA | Methylammonium |
| MAPbBr3 | MethylAmmonium Lead Bromide Perovskite |
| MAPbI3 | MethylAmmonium Lead Iodide Perovskite |
| MAPbI3-xClx | MethylAmmonium Lead Iodide/Chloride |
| m-TiO2 | Mesoporous titanium dioxide |
| NE | Non-Encapsulated devices |
| NMR | Nuclear Magnetic Resonance |
| OFET | Organic thin-film transistor |
| OLED | Organic Light Emitting Diode |
| OPV | Organic PhotoVoltaics |
| P | Maximum Power |
| Pin | Incident Power at Constant Flux of Photons |
| P3HT | Poly(3-hexyl)thiophene |
| PANI | Polyaniline |
| PCE | Power Conversion Efficiency |
| PDI | Polydispersity index |
| PEDOT:PSS | Poly(3,4-ethylenedioxythiophene) polystyrene sulfonate |
| PFN | Poly[(9,9-bis(3′-(N,N-dimethylamino)propyl)-2,7-fluorene)-alt-2,7-(9,9-dioctylfluorene)] |
| PPN | Poly(4,4′-bis(N-carbazolyl)-1,10-biphenyl) |
| PPP | Polyphenylene |
| PSC | Perovskite Solar Cell |
| PT | Polythiophene |
| PTAA | Poly TriArylAmine |
| QD | Quantum Dot |
| RH | Relative Humidity |
| Spiro-OMeTAD | 2,2′,7,7′-Tetrakis[N,N-di(4-methoxyphenyl)amino]-9,9′-spirobifluorene |
| tBP | 4-Tert-ButylPyridine |
| Voc | Open Circuit Voltage |
| VB | Valence Band |
References
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef] [PubMed]
- Carella, A.; Borbone, F.; Centore, R. Research Progress on Photosensitizers for DSSC. Front. Chem. 2018, 6, 481. [Google Scholar] [CrossRef] [PubMed]
- Nazeeruddin, M.K.; Snaith, H. Methylammonium lead triiodide perovskite solar cells: A new paradigm in photovoltaics. MRS Bull. 2015, 40, 641–645. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Lee, C.R.; Im, J.H.; Lee, K.B.; Moehl, T.; Marchioro, A.; Moon, S.J.; Humphry-Baker, R.; Yum, J.H.; Moser, J.E.; et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376. [Google Scholar] [CrossRef] [Green Version]
- NREL. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 6 May 2021).
- Calio, L.; Kazim, S.; Gratzel, M.; Ahmad, S. Hole-Transport Materials for Perovskite Solar Cells. Angew. Chem. Int. Ed. Engl. 2016, 55, 14522–14545. [Google Scholar] [CrossRef]
- Motta, C.; El-Mellouhi, F.; Sanvito, S. Charge carrier mobility in hybrid halide perovskites. Sci. Rep. 2015, 5, 12746. [Google Scholar] [CrossRef]
- Chandrasekhar, P. Conducting Polymers, Fundamentals and Applications, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2018; p. 850. [Google Scholar]
- Chen, J.; Park, N.-G. Causes and Solutions of Recombination in Perovskite Solar Cells. Adv. Mater. 2018, 31, 1803019. [Google Scholar] [CrossRef]
- Rakstys, K.; Igci, C.; Nazeeruddin, M.K. Efficiency vs. stability: Dopant-free hole transporting materials towards stabilized perovskite solar cells. Chem. Sci. 2019, 10, 6748–6769. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Wen, Z.; Gao, P. Less is More: Dopant-Free Hole Transporting Materials for High-Efficiency Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1702512. [Google Scholar] [CrossRef]
- Urieta-Mora, J.; Garcia-Benito, I.; Molina-Ontoria, A.; Martin, N. Hole transporting materials for perovskite solar cells: A chemical approach. Chem. Soc. Rev. 2018, 47, 8541–8571. [Google Scholar] [CrossRef] [PubMed]
- Ummadisingu, A.; Seo, J.Y.; Stojanovic, M.; Zakeeruddin, S.M.; Gratzel, M.; Hagfeldt, A.; Vlachopoulos, N.; Saliba, M. Additives, Hole Transporting Materials and Spectroscopic Methods to Characterize the Properties of Perovskite Films. Chimia 2017, 71, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.K.; Fincher, C.R.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; Gau, S.C.; MacDiarmid, A.G. Electrical Conductivity in Doped Polyacetylene. Phys. Rev. Lett. 1977, 39, 1098–1101. [Google Scholar] [CrossRef]
- Shahnawaz Sudheendran Swayamprabha, S.; Nagar, M.R.; Yadav, R.A.K.; Gull, S.; Dubey, D.K.; Jou, J.-H. Hole-transporting materials for organic light-emitting diodes: An overview. J. Mater. Chem. C 2019, 7, 7144–7158. [Google Scholar] [CrossRef]
- Quinn, J.T.E.; Zhu, J.; Li, X.; Wang, J.; Li, Y. Recent progress in the development of n-type organic semiconductors for organic field effect transistors. J. Mater. Chem. C 2017, 5, 8654–8681. [Google Scholar] [CrossRef]
- Garnier, F. Functionalized conducting polymers—Towards intelligent materials. Angew. Chem. 1989, 101, 529–533. [Google Scholar] [CrossRef]
- Foot, P.J.S.; Kaiser, A.B. Conducting Polymers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 513–550. [Google Scholar]
- Wessling, B. Conductive Polymers as Organic Nanometals; CRC Press LLC: Boca Raton, FL, USA, 2007; pp. 1/3–1/75. [Google Scholar]
- Magomedov, A.; Kasparavičius, E.; Rakstys, K.; Paek, S.; Gasilova, N.; Genevičius, K.; Juška, G.; Malinauskas, T.; Nazeeruddin, M.K.; Getautis, V. Pyridination of hole transporting material in perovskite solar cells questions the long-term stability. J. Mater. Chem. C 2018, 6, 8874–8878. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding Degradation Mechanisms and Improving Stability of Perovskite Photovoltaics. Chem Rev. 2019, 119, 3418–3451. [Google Scholar] [CrossRef]
- Desoky, M.M.H.; Bonomo, M.; Buscaino, R.; Fin, A.; Viscardi, G.; Barolo, C.; Quagliotto, P. Dopant-free All-Organic Small Molecule HTMs for Perovskite Solar Cells: Concepts and Structure-Property Relationships. Energies 2021, 14, 2279. [Google Scholar] [CrossRef]
- Quagliotto, P.; Fin, A. Advances in Synthetic Methods for the Preparation of Poly(3-hexylthiophene) (P3HT). Lett. Org. Chem. 2018, 15, 991–1006. [Google Scholar] [CrossRef]
- Tremel, K.; Ludwigs, S. Morphology of P3HT in Thin Films in Relation to Optical and Electrical Properties. In P3HT Revisited—From Molecular Scale to Solar Cell Devices; Ludwig, S., Ed.; Springr: Heidelberg, Germany, 2014; Volume 265, pp. 39–82. [Google Scholar]
- Maruo, H.; Sasaki, Y.; Harada, K.; Suwa, K.; Oyaizu, K.; Segawa, H.; Carter, K.; Nishide, H. Hole-transporting diketopyrrolopyrrole-thiophene polymers and their additive-free application for a perovskite-type solar cell with an efficiency of 16.3%. Polym. J. 2019, 51, 91–96. [Google Scholar] [CrossRef]
- Schopf, G.; Kossmehl, G. Polythiophenes—Electrically Conductive Polymers; Springer: Berlin/Heidelberg, Germany, 1997; Volume 129. [Google Scholar]
- Yaghoobi Nia, N.; Bonomo, M.; Zendehdel, M.; Lamanna, E.; Desoky, M.M.H.; Paci, B.; Zurlo, F.; Generosi, A.; Barolo, C.; Viscardi, G.; et al. Impact of P3HT Regioregularity and Molecular Weight on the Efficiency and Stability of Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2021, 9, 5061–5073. [Google Scholar] [CrossRef]
- De Rossi, F.; Renno, G.; Taheri, B.; Yaghoobi Nia, N.; Ilieva, V.; Fin, A.; Di Carlo, A.; Bonomo, M.; Barolo, C.; Brunetti, F. Modified P3HT materials as hole transport layers for flexible perovskite solar cells. J. Power Sources 2021, 494, 229735. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, D.; Li, Z.A. Recent advances in the design of dopant-free hole transporting materials for highly efficient perovskite solar cells. Chin. Chem. Lett. 2018, 29, 219–231. [Google Scholar] [CrossRef]
- Liu, D.; Yongsheng, L. Recent progress of dopant-free organic hole-transporting materials in perovskite solar cells. J. Semicond. 2017, 38, 011005. [Google Scholar]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Fan, Q.; Biesold-McGee, G.V.; Ma, J.; Xu, Q.; Pan, S.; Peng, J.; Lin, Z. Lead-Free Halide Perovskite Nanocrystals: Crystal Structures, Synthesis, Stabilities, and Optical Properties. Angew. Chem. Int. Ed. 2020, 59, 1030–1046. [Google Scholar] [CrossRef]
- Umari, P.; Mosconi, E.; De Angelis, F. Relativistic GW calculations on CH3NH3PbI3 and CH3NH3SnI3 perovskites for solar cell applications. Sci. Rep. 2014, 4, 4467. [Google Scholar] [CrossRef] [Green Version]
- Yusoff, A.R.B.M.; Nazeeruddin, M.K. Organohalide Lead Perovskites for Photovoltaic Applications. J. Phys. Chem. Lett. 2016, 7, 851–866. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, F.; Liu, X.; Sun, M.; Han, J.; You, J.; Wang, S.; Xiao, Y.; Li, X. Dopant-Free Hole-Transport Material with a Tetraphenylethene Core for Efficient Perovskite Solar Cells. Energy Technol. 2017, 5, 1257–1264. [Google Scholar] [CrossRef]
- Roy, A.; Ghosh, A.; Bhandari, S.; Sundaram, S.; Mallick, T.K. Perovskite Solar Cells for BIPV Application: A Review. Buildings 2020, 10, 129. [Google Scholar] [CrossRef]
- Knutson, J.L.; Martin, J.D.; Mitzi, D.B. Tuning the band gap in hybrid tin iodide perovskite semiconductors using structural templating. Inorg. Chem. 2005, 44, 4699–4705. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Solar cells. Electron-hole diffusion lengths > 175 mum in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014, 7, 982. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Gratzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron- and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, Y.; Li, Y. Readily synthesized dopant-free hole transport materials with phenol core for stabilized mixed perovskite solar cells. J. Power Sources 2017, 344, 160–169. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domanski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Wang, Z.-K.; Liao, L.-S. Progress of Triple Cation Organometal Halide Perovskite Solar Cells. Energy Technol. 2020, 8, 1900804. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Liu, F.Z.; Tam, H.W.; Wong, M.K.; Ng, A.; Surya, C.; Chen, W.; He, Z.B. Perovskite solar cells—An overview of critical issues. Prog. Quantum Electron. 2017, 53, 1–37. [Google Scholar] [CrossRef]
- Li, G.; Zhang, T.; Zhao, Y. Hydrochloric acid accelerated formation of planar CH3NH3PbI3 perovskite with high humidity tolerance. J. Mater. Chem. A 2015, 3, 19674–19678. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef]
- Ahn, N.; Son, D.Y.; Jang, I.H.; Kang, S.M.; Choi, M.; Park, N.G. Highly Reproducible Perovskite Solar Cells with Average Efficiency of 18.3% and Best Efficiency of 19.7% Fabricated via Lewis Base Adduct of Lead(II) Iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Pan, Y.; Wang, Z.; Xia, Y.; Chen, Y.; Huang, W. Additive engineering for highly efficient organic–inorganic halide perovskite solar cells: Recent advances and perspectives. J. Mater. Chem. A 2017, 5, 12602–12652. [Google Scholar] [CrossRef]
- Yin, J.; Qu, H.; Cao, J.; Tai, H.; Li, J.; Zheng, N. Vapor-assisted crystallization control toward high performance perovskite photovoltaics with over 18% efficiency in the ambient atmosphere. J. Mater. Chem. A 2016, 4, 13203–13210. [Google Scholar] [CrossRef]
- Leyden, M.R.; Ono, L.K.; Raga, S.R.; Kato, Y.; Wang, S.; Qi, Y. High performance perovskite solar cells by hybrid chemical vapor deposition. J. Mater. Chem. A 2014, 2, 18742–18745. [Google Scholar] [CrossRef] [Green Version]
- Schloemer, T.H.; Christians, J.A.; Luther, J.M.; Sellinger, A. Doping strategies for small molecule organic hole-transport materials: Impacts on perovskite solar cell performance and stability. Chem. Sci. 2019, 10, 1904–1935. [Google Scholar] [CrossRef] [Green Version]
- Tress, W.; Marinova, N.; Inganas, M.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Michael, G. The Role of the Hole-Transport Layer in Perovskite Solar Cells—Reducing Recombination and Increasing Absorption. In Proceedings of the 2014 IEEE 40th Photovoltaic Specialist Conference (PVSC), Denver, CO, USA, 8–13 June 2014; pp. 1563–1566. [Google Scholar]
- Berry, J.; Buonassisi, T.; Egger, D.A.; Hodes, G.; Kronik, L.; Loo, Y.L.; Lubomirsky, I.; Marder, S.R.; Mastai, Y.; Miller, J.S.; et al. Hybrid Organic-Inorganic Perovskites (HOIPs): Opportunities and Challenges. Adv. Mater. 2015, 27, 5102–5112. [Google Scholar] [CrossRef]
- Jung, H.S.; Han, G.S.; Park, N.-G.; Ko, M.J. Flexible Perovskite Solar Cells. Joule 2019, 3, 1850–1880. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, A.; Cai, F.; Tao, L.; Zhou, Y.; Zhao, Z.; Chen, Q.; Cheng, Y.-B.; Zhou, H. Nickel oxide nanoparticles for efficient hole transport in p-i-n and n-i-p perovskite solar cells. J. Mater. Chem. A 2017, 5, 6597–6605. [Google Scholar] [CrossRef]
- Momblona, C.; Gil-Escrig, L.; Bandiello, E.; Hutter, E.M.; Sessolo, M.; Lederer, K.; Blochwitz-Nimoth, J.; Bolink, H.J. Efficient vacuum deposited p-i-n and n-i-p perovskite solar cells employing doped charge transport layers. Energy Environ. Sci. 2016, 9, 3456–3463. [Google Scholar] [CrossRef]
- Li, M.-H.; Shen, P.-S.; Wang, K.-C.; Guo, T.-F.; Chen, P. Inorganic p-type contact materials for perovskite-based solar cells. J. Mater. Chem. A 2015, 3, 9011–9019. [Google Scholar] [CrossRef]
- Cetin, C.; Chen, P.; Hao, M.; He, D.; Bai, Y.; Lyu, M.; Yun, J.-H.; Wang, L. Inorganic p-Type Semiconductors as Hole Conductor Building Blocks for Robust Perovskite Solar Cells. Adv. Sustain. Syst. 2018, 2, 1800032. [Google Scholar] [CrossRef]
- MacDiarmid, A.G. “Synthetic metals”: A novel role for organic polymers (Nobel Lecture). Angew. Chem. Int. Ed. 2001, 40, 2581–2590. [Google Scholar] [CrossRef]
- Heeger, A.J. Semiconducting and metallic polymers: The fourth generation of polymeric materials. Synth. Met. 2001, 125, 23–42. [Google Scholar] [CrossRef]
- Fu, K.; Wing Yi Ho-Baillie, A.; Kumar Mulmudi, H.; Trang, P.T.T. Perovskite Solar Cells: Technology and Practices; Apple Academic Press: Boca Raton, FL, USA, 2019; p. 332. [Google Scholar]
- Schloemer, T.H.; Gehan, T.S.; Christians, J.A.; Mitchell, D.G.; Dixon, A.; Li, Z.; Zhu, K.; Berry, J.J.; Luther, J.M.; Sellinger, A. Thermally Stable Perovskite Solar Cells by Systematic Molecular Design of the Hole-Transport Layer. ACS Energy Lett. 2019, 4, 473–482. [Google Scholar] [CrossRef]
- Tsai, C.-E.; Liao, M.-H.; Chen, Y.-L.; Cheng, S.-W.; Lai, Y.-Y.; Cheng, Y.-J.; Hsu, C.-S. Triarylamine-based crosslinked hole-transporting material with an ionic dopant for high-performance PEDOT:PSS-free polymer solar cells. J. Mater. Chem. C 2015, 3, 6158–6165. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Noel, N.K.; Snaith, H.J.; Nicholas, R.J. Investigating the Role of 4-TertButylpyridine in Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1601079. [Google Scholar] [CrossRef]
- Yang, J.; Siempelkamp, B.D.; Liu, D.; Kelly, T.L. Investigation of CH3NH3PbI3 degradation rates and mechanisms in controlled humidity environments using in situ techniques. ACS Nano 2015, 9, 1955–1963. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, C.; Yang, Y.; Harvey, S.P.; Kim, D.H.; Christians, J.A.; Yang, M.; Schulz, P.; Nanayakkara, S.U.; Jiang, C.-S.; et al. Extrinsic ion migration in perovskite solar cells. Energy Environ. Sci. 2017, 10, 1234–1242. [Google Scholar] [CrossRef]
- Yue, Y.; Salim, N.; Wu, Y.; Yang, X.; Islam, A.; Chen, W.; Liu, J.; Bi, E.; Xie, F.; Cai, M.; et al. Enhanced Stability of Perovskite Solar Cells through Corrosion-Free Pyridine Derivatives in Hole-Transporting Materials. Adv. Mater. 2016, 28, 10738–10743. [Google Scholar] [CrossRef] [PubMed]
- Snaith, H.J.; Grätzel, M. Electron and Hole Transport through Mesoporous TiO2 Infiltrated with Spiro-MeOTAD. Adv. Mater. 2007, 19, 3643–3647. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Qin, C.; Yang, X.; Yasuda, T.; Islam, A.; Zhang, K.; Peng, W.; Chen, W.; Han, L. A dopant-free hole-transporting material for efficient and stable perovskite solar cells. Energy Environ. Sci. 2014, 7, 2963–2967. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Duan, H.-S.; Zhou, H.; Yang, Y.; Chen, H.; Luo, S.; Song, T.-B.; Dou, L.; Hong, Z.; et al. A dopant-free organic hole transport material for efficient planar heterojunction perovskite solar cells. J. Mater. Chem. A 2015, 3, 11940–11947. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Wang, L.; Tu, B.; Chen, T.; Liu, B.; Yang, K.; Koh, C.W.; Zhang, X.; Sun, H.; et al. Dopant-Free Small-Molecule Hole-Transporting Material for Inverted Perovskite Solar Cells with Efficiency Exceeding 21. Adv. Mater. 2019, 31, e1902781. [Google Scholar] [CrossRef]
- Hou, W.; Xiao, Y.; Han, G.; Lin, J.Y. The Applications of Polymers in Solar Cells: A Review. Polymers 2019, 11, 143. [Google Scholar] [CrossRef] [Green Version]
- Kimpel, J.; Michinobu, T. Conjugated polymers for functional applications: Lifetime and performance of polymeric organic semiconductors in organic field-effect transistors. Polym. Int. 2020, 70, 367–373. [Google Scholar] [CrossRef]
- Alsalhi, M.S.; Alam, J.; Dass, L.A.; Raja, M. Recent advances in conjugated polymers for light emitting devices. Int. J. Mol. Sci 2011, 12, 2036–2054. [Google Scholar] [CrossRef]
- Cai, B.; Xing, Y.; Yang, Z.; Zhang, W.-H.; Qiu, J. High performance hybrid solar cells sensitized by organolead halide perovskites. Energy Environ. Sci. 2013, 6, 1480–1485. [Google Scholar] [CrossRef]
- Heo, J.H.; Han, H.J.; Kim, D.; Ahn, T.K.; Im, S.H. Hysteresis-less inverted CH3NH3PbI3 planar perovskite hybrid solar cells with 18.1% power conversion efficiency. Energy Environ. Sci. 2015, 8, 1602–1608. [Google Scholar] [CrossRef]
- Jung, E.H.; Jeon, N.J.; Park, E.Y.; Moon, C.S.; Shin, T.J.; Yang, T.Y.; Noh, J.H.; Seo, J. Efficient, stable and scalable perovskite solar cells using poly(3-hexylthiophene). Nature 2019, 567, 511–515. [Google Scholar] [CrossRef]
- Tepliakova, M.M.; Akkuratov, A.V.; Tsarev, S.A.; Troshin, P.A. Suzuki polycondensation for the synthesis of polytriarylamines: A method to improve hole-transport material performance in perovskite solar cells. Tetrahedron Lett. 2020, 61, 152317. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, G.; Jeon, N.J.; Lim, C.; Seo, J.; Kim, B.J. Methoxy-Functionalized Triarylamine-Based Hole-Transporting Polymers for Highly Efficient and Stable Perovskite Solar Cells. ACS Energy Lett. 2020, 5, 3304–3313. [Google Scholar] [CrossRef]
- Lu, H.; Ma, Y.; Gu, B.; Tian, W.; Li, L. Identifying the optimum thickness of electron transport layers for highly efficient perovskite planar solar cells. J. Mater. Chem. A 2015, 3, 16445–16452. [Google Scholar] [CrossRef]
- Li, M.-H.; Liu, S.-C.; Qiu, F.-Z.; Zhang, Z.-Y.; Xue, D.-J.; Hu, J.-S. High-Efficiency CsPbI2Br Perovskite Solar Cells with Dopant-Free Poly(3-hexylthiophene) Hole Transporting Layers. Adv. Energy Mater. 2020, 10, 2000501. [Google Scholar] [CrossRef]
- Zhang, W.; Wan, L.; Li, X.; Wu, Y.; Fu, S.; Fang, J. A dopant-free polyelectrolyte hole-transport layer for high efficiency and stable planar perovskite solar cells. J. Mater. Chem. A 2019, 7, 18898–18905. [Google Scholar] [CrossRef]
- Zhang, W.; Wan, L.; Fu, S.; Li, X.; Fang, J. Reducing energy loss and stabilizing the perovskite/poly(3-hexylthiophene) interface through a polyelectrolyte interlayer. J. Mater. Chem. A 2020, 8, 6546–6554. [Google Scholar] [CrossRef]
- Huan, Y.; Tan, C.; Wu, B.; Feng, X.; Xu, W.; Gao, D. A Dopant-Free Zwitterionic Conjugated Polyelectrolyte as a Hole-Transporting and Interfacial Material for Perovskite Solar Cells. Sol. RRL 2020, 4, 2000206. [Google Scholar] [CrossRef]
- Han, S.; Jiang, X.; Yu, Z.; Wan, X.; Zang, J.; Zhang, C.; Rui, H.; Yang, X.; Hagfeldt, A.; Sun, L. Side-chain engineering of PEDOT derivatives as dopant-free hole-transporting materials for efficient and stable n-i-p structured perovskite solar cells. J. Mater. Chem. C 2020, 8, 9236–9242. [Google Scholar] [CrossRef]
- Xiao, Y.; Han, G.; Chang, Y.; Zhou, H.; Li, M.; Li, Y. An all-solid-state perovskite-sensitized solar cell based on the dual function polyaniline as the sensitizer and p-type hole-transporting material. J. Power Sources 2014, 267, 1–8. [Google Scholar] [CrossRef]
- Yan, W.; Li, Y.; Li, Y.; Ye, S.; Liu, Z.; Wang, S.; Bian, Z.; Huang, C. High-performance hybrid perovskite solar cells with open circuit voltage dependence on hole-transporting materials. Nano Energy 2015, 16, 428–437. [Google Scholar] [CrossRef]
- Xu, Y.; Bu, T.; Li, M.; Qin, T.; Yin, C.; Wang, N.; Li, R.; Zhong, J.; Li, H.; Peng, Y.; et al. Non-Conjugated Polymer as an Efficient Dopant-Free Hole-Transporting Material for Perovskite Solar Cells. ChemSusChem 2017, 10, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Ji, R.; Xu, W.; Yin, C.; Wen, K.; Gao, H.; Yang, R.; Pan, Z.; Wang, K.; Zhang, C.; et al. Non-Conjugated Polymer Based on Polyethylene Backbone as Dopant-Free Hole-Transporting Material for Efficient and Stable Inverted Quasi-2D Perovskite Solar Cells. Sol. RRL 2020, 4, 2000184. [Google Scholar] [CrossRef]
- Wu, J.; Liu, C.; Li, B.; Gu, F.; Zhang, L.; Hu, M.; Deng, X.; Qiao, Y.; Mao, Y.; Tan, W.; et al. Side-Chain Polymers as Dopant-Free Hole-Transporting Materials for Perovskite Solar Cells-The Impact of Substituents′ Positions in Carbazole on Device Performance. ACS Appl. Mater. Interfaces 2019, 11, 26928–26937. [Google Scholar] [CrossRef]
- Lan, L.; Deng, X.; Zhang, J.; Luo, J.; Jen, A.K.Y. Synthesis of a side-chain hole transporting polymer through Mitsunobu post-functionalization for efficient inverted perovskite solar cells. Polym. Chem. 2020, 11, 2883–2888. [Google Scholar] [CrossRef]
- Tremblay, M.-H.; Schutt, K.; Zhang, Y.; Lim, J.; Lin, Y.-H.; Warby, J.H.; Barlow, S.; Snaith, H.J.; Marder, S.R. A photo-crosslinkable bis-triarylamine side-chain polymer as a hole-transport material for stable perovskite solar cells. Sustain. Energy Fuels 2020, 4, 190–198. [Google Scholar] [CrossRef]
- Zhang, Y.; Kou, C.; Zhang, J.; Liu, Y.; Li, W.; Bo, Z.; Shao, M. Crosslinked- and dopant-free hole transport materials for efficient and stable planar perovskite solar cells. J. Mater. Chem. A 2019, 7, 5522–5529. [Google Scholar] [CrossRef]
- Vaitukaityte, D.; Al-Ashouri, A.; Daskeviciene, M.; Kamarauskas, E.; Nekrasovas, J.; Jankauskas, V.; Magomedov, A.; Albrecht, S.; Getautis, V. Enamine-Based CrossLinkable Hole-Transporting Materials for Perovskite Solar Cells. Sol. RRL 2021, 5, 2000597. [Google Scholar] [CrossRef]
- Wu, J.; Liu, C.; Hu, M.; Deng, X.; Tan, W.; Tian, Y.; Xu, B. Polystyrene with a methoxytriphenylamine-conjugated-thiophene moiety side-chain as a dopant-free hole-transporting material for perovskite solar cells. J. Mater. Chem. A 2018, 6, 13123–13132. [Google Scholar] [CrossRef]
- Ruankham, P.; Sagawa, T. Dopant-free π-conjugated polymers as hole-transporting materials for stable perovskite solar cells. J. Mater. Sci.: Mater. Electron. 2018, 29, 9058–9066. [Google Scholar] [CrossRef]
- Lin, Q.; Armin, A.; Nagiri, R.C.R.; Burn, P.L.; Meredith, P. Electro-optics of perovskite solar cells. Nat. Photonics 2014, 9, 106. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, B.; Lin, Z.; Su, J.; Yang, Z.; Zhang, C.; Chang, J.; Liu, S.; Hao, Y. Highly efficient perovskite solar cells based on a dopant-free conjugated DPP polymer hole transport layer: Influence of solvent vapor annealing. Sustain. Energy Fuels 2018, 2, 2154–2159. [Google Scholar] [CrossRef]
- Dubey, A.; Adhikari, N.; Venkatesan, S.; Gu, S.; Khatiwada, D.; Wang, Q.; Mohammad, L.; Kumar, M.; Qiao, Q. Solution processed pristine PDPP3T polymer as hole transport layer for efficient perovskite solar cells with slower degradation. Sol. Energy Mater. Sol. Cells 2016, 145, 193–199. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Lim, J.; Yun, H.-J.; Kim, Y.-H.; Park, T. A diketopyrrolopyrrole-containing hole transporting conjugated polymer for use in efficient stable organic–inorganic hybrid solar cells based on a perovskite. Energy Environ. Sci. 2014, 7, 1454–1460. [Google Scholar] [CrossRef]
- Kang, I.; An, T.K.; Hong, J.-A.; Yun, H.-J.; Kim, R.; Chung, D.S.; Park, C.E.; Kim, Y.-H.; Kwon, S.-K. Effect of Selenophene in a DPP Copolymer Incorporating a Vinyl Group for High-Performance Organic Field-Effect Transistors. Adv. Mater. 2013, 25, 524–528. [Google Scholar] [CrossRef]
- Liu, J.; Ge, Q.Q.; Zhang, W.F.; Ma, J.Y.; Ding, J.; Yu, G.; Hu, J.S. Highly pi-extended copolymer as additive-free hole-transport material for perovskite solar cells. Nano Res. 2018, 11, 185–194. [Google Scholar] [CrossRef]
- Kulshreshtha, C.; Clement, A.; Pascher, T.; Sundstroem, V.; Matyba, P. Investigating ultrafast carrier dynamics in perovskite solar cells with an extended π-conjugated polymeric diketopyrrolopyrrole layer for hole transportation. RSC Adv. 2020, 10, 6618–6624. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Ma, Y.; Wang, Z.; Zhu, M.; Wang, J.; Khalil, M.; Wang, H.; Gao, W.; Fan, W.J.; Li, W.-S.; et al. Improving the Fill Factor of Perovskite Solar Cells by Employing an Amine-tethered Diketopyrrolopyrrole-Based Polymer as the Dopant-free Hole Transport Layer. ACS Appl. Energy Mater. 2020, 3, 9600–9609. [Google Scholar] [CrossRef]
- Liu, W.; Ma, Y.; Wang, Z.; Mu, Z.; Gao, W.; Fan, W.; Li, W.-S.; Zhang, Q. Improving the hole transport performance of perovskite solar cells through adjusting the mobility of the as-synthesized conjugated polymer. J. Mater. Chem. C 2021, 9, 3421–3428. [Google Scholar] [CrossRef]
- Fu, Q.; Xu, Z.; Tang, X.; Liu, T.; Dong, X.; Zhang, X.; Zheng, N.; Xie, Z.; Liu, Y. Multifunctional Two-Dimensional Conjugated Materials for Dopant-Free Perovskite Solar Cells with Efficiency Exceeding 22%. ACS Energy Lett. 2021, 6, 1521–1532. [Google Scholar] [CrossRef]
- Beimling, P.; Kobmehl, G. Synthesis of benzo[1,2-b:4,5-b′]dithiophene and its 4,8-dimethoxy and 4,8-dimethyl derivatives. Chem. Ber. 1986, 119, 3198–3203. [Google Scholar] [CrossRef]
- Pan, H.; Li, Y.; Wu, Y.; Liu, P.; Ong, B.S.; Zhu, S.; Xu, G. Low-temperature, solution-processed, high-mobility polymer semiconductors for thin-film transistors. J. Am. Chem. Soc. 2007, 129, 4112–4113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hau, S.K.; Yip, H.-L.; Sun, Y.; Acton, O.; Jen, A.K.Y. Efficient Polymer Solar Cells Based on the Copolymers of Benzodithiophene and Thienopyrroledione. Chem. Mater. 2010, 22, 2696–2698. [Google Scholar] [CrossRef]
- Chen, L.; Shen, X.; Chen, Y. A Novel Thiophene Derivative-based Conjugated Polymer for Polymer Solar Cells with High Open-circuit Voltage. Chin. J. Chem. 2012, 30, 2219–2224. [Google Scholar] [CrossRef]
- Busireddy, M.R.; Mantena, V.N.R.; Chereddy, N.R.; Shanigaram, B.; Kotamarthi, B.; Biswas, S.; Sharma, G.D.; Vaidya, J.R. Dithienopyrrole-benzodithiophene based donor materials for small molecular BHJSCs: Impact of side chain and annealing treatment on their photovoltaic properties. Org. Electron. 2016, 37, 312–325. [Google Scholar] [CrossRef]
- Zhou, J.; Zuo, Y.; Wan, X.; Long, G.; Zhang, Q.; Ni, W.; Liu, Y.; Li, Z.; He, G.; Li, C.; et al. Solution-processed and high-performance organic solar cells using small molecules with a benzodithiophene unit. J. Am. Chem. Soc. 2013, 135, 8484–8487. [Google Scholar] [CrossRef]
- Kim, G.W.; Lee, J.; Kang, G.; Kim, T.; Park, T. Donor-Acceptor Type Dopant-Free, Polymeric Hole Transport Material for Planar Perovskite Solar Cells (19.8%). Adv. Energy Mater. 2018, 8, 1701935. [Google Scholar] [CrossRef]
- Lee, J.; Kim, G.-W.; Kim, M.; Park, S.A.; Park, T. Nonaromatic Green-Solvent-Processable, Dopant-Free, and Lead-Capturable Hole Transport Polymers in Perovskite Solar Cells with High Efficiency. Adv. Energy Mater. 2020, 10, 1902662. [Google Scholar] [CrossRef]
- Marin-Beloqui, J.M.; Hernández, J.P.; Palomares, E. Photo-induced charge recombination kinetics in MAPbI3−xClx perovskite-like solar cells using low band-gap polymers as hole conductors. Chem. Commun. 2014, 50, 14566–14569. [Google Scholar] [CrossRef]
- Kim, J.; Kim, G.; Kim, T.K.; Kwon, S.; Back, H.; Lee, J.; Lee, S.H.; Kang, H.; Lee, K. Efficient planar-heterojunction perovskite solar cells achieved via interfacial modification of a sol–gel ZnO electron collection layer. J. Mater. Chem. A 2014, 2, 17291–17296. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, X.; Zhang, J.; Ahmad, S.; Tu, D.; Qin, W.; Jiu, T.; Pang, S.; Guo, X.; Li, C. Simultaneous hole transport and defect passivation enabled by a dopant-free single polymer for efficient and stable perovskite solar cells. J. Mater. Chem. A 2020, 8, 21036–21043. [Google Scholar] [CrossRef]
- Liu, X.; Fu, P.; Tu, D.; Yang, Q.; Yu, S.; Guo, X.; Li, C. Bifunctional donor polymers bearing amino pendant groups for efficient cathode interlayer-free polymer solar cells. J. Mater. Chem. A 2018, 6, 19828–19833. [Google Scholar] [CrossRef]
- Murugesan, V.S.; Michael, R.R.; Jena, A.K.; Kang, J.-W.; Kim, N.H.; Segawa, H.; Miyasaka, T.; Lee, J.H. Benzodithiophene-thienopyrroledione-thienothiophene-based random copolymeric hole transporting material for perovskite solar cell. Chem. Eng. J. 2020, 382, 122830. [Google Scholar] [CrossRef]
- Yuan, J.; Ling, X.; Yang, D.; Li, F.; Zhou, S.; Shi, J.; Qian, Y.; Hu, J.; Sun, Y.; Yang, Y.; et al. Band-Aligned Polymeric Hole Transport Materials for Extremely Low Energy Loss α-CsPbI3 Perovskite Nanocrystal Solar Cells. Joule 2018, 2, 2450–2463. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-W.; Park, S.; Ko, M.J.; Son, H.J.; Park, N.-G. Enhancement of the Photovoltaic Performance of CH3NH3PbI3 Perovskite Solar Cells through a Dichlorobenzene-Functionalized Hole-Transporting Material. ChemPhysChem 2014, 15, 2595–2603. [Google Scholar] [CrossRef]
- Chen, W.; Bao, X.; Zhu, Q.; Zhu, D.; Qiu, M.; Sun, M.; Yang, R. Simple planar perovskite solar cells with a dopant-free benzodithiophene conjugated polymer as hole transporting material. J. Mater. Chem. C 2015, 3, 10070–10073. [Google Scholar] [CrossRef]
- Nagarjuna, P.; Narayanaswamy, K.; Swetha, T.; Rao, G.H.; Singh, S.P.; Sharma, G.D. CH3NH3PbI3 Perovskite Sensitized Solar Cells Using a D-A Copolymer as Hole Transport Material. Electrochim. Acta 2015, 151, 21–26. [Google Scholar] [CrossRef]
- Kim, G.-W.; Kang, G.; Kim, J.; Lee, G.-Y.; Kim, H.I.; Pyeon, L.; Lee, J.; Park, T. Dopant-free polymeric hole transport materials for highly efficient and stable perovskite solar cells. Energy Environ. Sci. 2016, 9, 2326–2333. [Google Scholar] [CrossRef]
- Kranthiraja, K.; Gunasekar, K.; Kim, H.; Cho, A.-N.; Park, N.-G.; Kim, S.; Kim, B.J.; Nishikubo, R.; Saeki, A.; Song, M.; et al. High-Performance Long-Term-Stable Dopant-Free Perovskite Solar Cells and Additive-Free Organic Solar Cells by Employing Newly Designed Multirole π-Conjugated Polymers. Adv. Mater. 2017, 29, 1700183. [Google Scholar] [CrossRef]
- Lee, J.; Malekshahi Byranvand, M.; Kang, G.; Son, S.Y.; Song, S.; Kim, G.-W.; Park, T. Green-Solvent-Processable, Dopant-Free Hole-Transporting Materials for Robust and Efficient Perovskite Solar Cells. J. Am. Chem. Soc. 2017, 139, 12175–12181. [Google Scholar] [CrossRef]
- Liao, H.-C.; Tam, T.L.D.; Guo, P.; Wu, Y.; Manley, E.F.; Huang, W.; Zhou, N.; Soe, C.M.M.; Wang, B.; Wasielewski, M.R.; et al. Dopant-Free Hole Transporting Polymers for High Efficiency, Environmentally Stable Perovskite Solar Cells. Adv. Energy Mater. 2016, 6, 1600502. [Google Scholar] [CrossRef]
- Kranthiraja, K.; Park, S.H.; Kim, H.; Gunasekar, K.; Han, G.; Kim, B.J.; Kim, C.S.; Kim, S.; Lee, H.; Nishikubo, R.; et al. Accomplishment of Multifunctional π-Conjugated Polymers by Regulating the Degree of Side-Chain Fluorination for Efficient Dopant-Free Ambient-Stable Perovskite Solar Cells and Organic Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 36053–36060. [Google Scholar] [CrossRef]
- Kranthiraja, K.; Arivunithi, V.M.; Aryal, U.K.; Park, H.-Y.; Cho, W.; Kim, J.; Reddy, S.S.; Kim, H.-K.; Kang, I.-N.; Song, M.; et al. Efficient and hysteresis-less perovskite and organic solar cells by employing donor-acceptor type π-conjugated polymer. Org. Electron. 2019, 72, 18–24. [Google Scholar] [CrossRef]
- Kyeong, M.; Lee, J.; Lee, K.; Hong, S. BODIPY-based conjugated polymers for use as dopant-free hole transporting materials for durable perovskite solar cells: Selective tuning of HOMO/LUMO levels. ACS Appl. Mater. Interfaces 2018, 10, 23254–23262. [Google Scholar] [CrossRef]
- Rana, P.J.S.; Gunasekaran, R.K.; Park, S.H.; Tamilavan, V.; Karuppanan, S.; Kim, H.-J.; Prabakar, K. Open Atmosphere-Processed Stable Perovskite Solar Cells Using Molecular Engineered, Dopant-Free, Highly Hydrophobic Polymeric Hole-Transporting Materials: Influence of Thiophene and Alkyl Chain on Power Conversion Efficiency. J. Phys. Chem. C 2019, 123, 8560–8568. [Google Scholar] [CrossRef]
- Qi, F.; Deng, X.; Wu, X.; Huo, L.; Xiao, Y.; Lu, X.; Zhu, Z.; Jen, A.K.Y. A Dopant-Free Polymeric Hole-Transporting Material Enabled High Fill Factor Over 81% for Highly Efficient Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1902600. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Jeong, M.; Lee, S.M.; Du, B.; Mao, Y.; Ye, F.; Zhang, H.; Li, D.; Liu, D.; et al. Dopant-free polymeric hole transport materials for efficient CsPbI2Br perovskite cells with a fill factor exceeding 84%. J. Mater. Chem. C 2020, 8, 8507–8514. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, Z.; Guo, Y.; Li, Y.; Bergstrand, J.; Brett, C.J.; Cai, B.; Hajian, A.; Guo, Y.; Yang, X.; et al. Polymeric, Cost-Effective, Dopant-Free Hole Transport Materials for Efficient and Stable Perovskite Solar Cells. J. Am. Chem. Soc. 2019, 141, 19700–19707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, J.; Li, D.; Li, W.; Meng, Q.; Bo, Z. Ladder-like conjugated polymers used as hole-transporting materials for high-efficiency perovskite solar cells. J. Mater. Chem. A 2019, 7, 14473–14477. [Google Scholar] [CrossRef]
- Kong, X.; Jiang, Y.; Wu, X.; Chen, C.; Guo, J.; Liu, S.; Gao, X.; Zhou, G.; Liu, J.-M.; Kempa, K.; et al. Dopant-free F-substituted benzodithiophene copolymer hole-transporting materials for efficient and stable perovskite solar cells. J. Mater. Chem. A 2020, 8, 1858–1864. [Google Scholar] [CrossRef]
- Firdaus, Y.; Maffei, L.P.; Cruciani, F.; Müller, M.A.; Liu, S.; Lopatin, S.; Wehbe, N.; Ndjawa, G.O.N.; Amassian, A.; Laquai, F.; et al. Polymer Main-Chain Substitution Effects on the Efficiency of Nonfullerene BHJ Solar Cells. Adv. Energy Mater. 2017, 7, 1700834. [Google Scholar] [CrossRef]
- Kappe, C.O.; Pieber, B.; Dallinger, D. Microwave effects in organic synthesis: Myth or reality? Angew. Chem. Int. Ed. Engl. 2013, 52, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Nagahata, R.; Takeuchi, K. Encouragements for the Use of Microwaves in Industrial Chemistry. Chem. Rec. 2018, 19, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.W.; Choi, H.; Kim, M.; Lee, J.; Son, S.Y.; Park, T. Hole Transport Materials in Conventional Structural (n–i–p) Perovskite Solar Cells: From Past to the Future. Adv. Energy Mater. 2020, 10, 1903403. [Google Scholar] [CrossRef]
- Liu, Y.; Hong, Z.; Chen, Q.; Chen, H.; Chang, W.-H.; Yang, Y.; Song, T.-B.; Yang, Y. Perovskite Solar Cells Employing Dopant-Free Organic Hole Transport Materials with Tunable Energy Levels. Adv. Mater. 2016, 28, 440–446. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Z.; Kuo, M.Y.; Chueh, C.C.; Jen, A.K. Hexaazatrinaphthylene Derivatives: Efficient Electron-Transporting Materials with Tunable Energy Levels for Inverted Perovskite Solar Cells. Angew. Chem. Int. Ed. Engl. 2016, 55, 8999–9003. [Google Scholar] [CrossRef]
- Song, Y.; Lv, S.; Liu, X.; Li, X.; Wang, S.; Wei, H.; Li, D.; Xiao, Y.; Meng, Q. Energy level tuning of TPB-based hole-transporting materials for highly efficient perovskite solar cells. Chem. Commun. 2014, 50, 15239–15242. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.; Liang, M.; Liu, L.; Wang, X.; Sun, Z.; Xue, S. Low-Cost Carbazole-Based Hole-Transporting Materials for Perovskite Solar Cells: Influence of S,N-Heterocycle. J. Phys. Chem. C 2018, 122, 24014–24024. [Google Scholar] [CrossRef]
- Gao, L.; Schloemer, T.H.; Zhang, F.; Chen, X.; Xiao, C.; Zhu, K.; Sellinger, A. Carbazole-Based Hole-Transport Materials for High-Efficiency and Stable Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 4492–4498. [Google Scholar] [CrossRef]
- Wu, F.; Ji, Y.; Wang, R.; Shan, Y.; Zhu, L. Molecular engineering to enhance perovskite solar cell performance: Incorporation of benzothiadiazole as core unit for low cost hole transport materials. Dyes Pigm. 2017, 143, 356–360. [Google Scholar] [CrossRef]
- Rodriguez-Seco, C.; Mendez, M.; Roldan-Carmona, C.; Cabau, L.; Asiri, A.M.; Nazeeruddin, M.K.; Palomares, E. Benzothiadiazole Aryl-amine Based Materials as Efficient Hole Carriers in Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 32712–32718. [Google Scholar] [CrossRef]
- Cai, F.; Cai, J.; Yang, L.; Li, W.; Gurney, R.S.; Yi, H.; Iraqi, A.; Liu, D.; Wang, T. Molecular engineering of conjugated polymers for efficient hole transport and defect passivation in perovskite solar cells. Nano Energy 2018, 45, 28–36. [Google Scholar] [CrossRef]
- Wong-Stringer, M.; Bishop, J.E.; Smith, J.A.; Mohamad, D.K.; Parnell, A.J.; Kumar, V.; Rodenburg, C.; Lidzey, D.G. Efficient perovskite photovoltaic devices using chemically doped PCDTBT as a hole-transport material. J. Mater. Chem. A 2017, 5, 15714–15723. [Google Scholar] [CrossRef]
- Liu, P.-H.; Chuang, C.-H.; Zhou, Y.-L.; Wang, S.-H.; Jeng, R.-J.; Rwei, S.-P.; Liau, W.-B.; Wang, L. Conjugated polyelectrolytes as promising hole transport materials for inverted perovskite solar cells: Effect of ionic groups. J. Mater. Chem. A 2020, 8, 25173–25177. [Google Scholar] [CrossRef]
- Kim, G.-W.; Kim, J.; Lee, G.-Y.; Kang, G.; Lee, J.; Park, T. A Strategy to Design a Donor–π–Acceptor Polymeric Hole Conductor for an Efficient Perovskite Solar Cell. Adv. Energy Mater. 2015, 5, 1500471. [Google Scholar] [CrossRef]
- Mahesh, K.; Karpagam, S.; Putnin, T.; Le, H.; Bui, T.-T.; Ounnunkad, K.; Goubard, F. Role of cyano substituents on thiophene vinylene benzothiadiazole conjugated polymers and application as hole transporting materials in perovskite solar cells. J. Photochem. Photobiol. A 2019, 371, 238–247. [Google Scholar] [CrossRef]
- Zhang, Z.; Sheri, M.; Page, Z.A.; Emrick, T.; Saeki, A.; Liu, Y.; Russell, T.P. Understanding Hole Extraction of Inverted Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 56068–56075. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Po, R.; Bianchi, G.; Carbonera, C.; Kong, J. Efficient and Stable Mesoscopic Perovskite Solar Cells Using a Dopant-Free D-A Copolymer Hole-Transporting Layer. Sol. RRL 2021, 5, 2000801. [Google Scholar] [CrossRef]
- Ma, H.; Yuan, L.; Chen, Q.; Fu, J.; Zhang, J.; Jiang, Z.; Dong, B.; Zhou, Y.; Yin, S.; Song, B. Conjugated copolymers as doping- and annealing-free hole transport materials for highly stable and efficient p-i-n perovskite solar cells. J. Mater. Chem. A 2021, 9, 2269–2275. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Chen, Q.; Liu, S.; Yun, Y.; Liu, Y.; Chen, C.; Wang, J.; Cao, Y.; Wang, F.; et al. Dopant-Free Hole-Transporting Polycarbazoles with Tailored Backbones for Efficient Inverted Perovskite Solar Cells. Macromolecules 2019, 52, 4757–4764. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, F.; Guo, Y.; Wu, H.; He, L.; Liu, Z.; Cai, B.; Guo, Y.; Brett, C.J.; Li, Y.; et al. Conformational and compositional tuning of phenanthrocarbazole-based dopant-free hole-transport polymers boosting performance of perovskite solar cells. J. Am. Chem. Soc. 2020, 142, 17681–17692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liang, L.; Deng, L.; Ren, L.; Zhao, N.; Huang, J.; Yu, Y.; Gao, P. Fused Dithienopicenocarbazole Enabling High Mobility Dopant-Free Hole-Transporting Polymers for Efficient and Stable Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 6688–6698. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Greenham, N.C. Charge mobility measurement techniques in organic semiconductors. Opt. Quantum Electron. 2009, 41, 69–89. [Google Scholar] [CrossRef]
- Ryu, S.; Noh, J.H.; Jeon, N.J.; Chan Kim, Y.; Yang, W.S.; Seo, J.; Seok, S.I. Voltage output of efficient perovskite solar cells with high open-circuit voltage and fill factor. Energy Environ. Sci. 2014, 7, 2614–2618. [Google Scholar] [CrossRef]
- Xiao, Q.; Tian, J.; Xue, Q.; Wang, J.; Xiong, B.; Han, M.; Li, Z.; Zhu, Z.; Yip, H.-L.; Li, Z.A. Dopant-Free Squaraine-Based Polymeric Hole-Transporting Materials with Comprehensive Passivation Effects for Efficient All-Inorganic Perovskite Solar Cells. Angew. Chem. Int. Ed. 2019, 58, 17724–17730. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Kan, M.; Zhao, Y. Bifunctional Stabilization of All-Inorganic α-CsPbI3 Perovskite for 17% Efficiency Photovoltaics. J. Am. Chem. Soc. 2018, 140, 12345–12348. [Google Scholar] [CrossRef]
- You, G.; Zhuang, Q.; Wang, L.; Lin, X.; Zou, D.; Lin, Z.; Zhen, H.; Zhuang, W.; Ling, Q. Dopant-Free, Donor-Acceptor-Type Polymeric Hole-Transporting Materials for the Perovskite Solar Cells with Power Conversion Efficiencies over 20%. Adv. Energy Mater. 2020, 10, 1903146. [Google Scholar] [CrossRef]
- Chawanpunyawat, T.; Funchien, P.; Wongkaew, P.; Henjongchom, N.; Ariyarit, A.; Ittisanronnachai, S.; Namuangruk, S.; Cheacharoen, R.; Sudyoadsuk, T.; Goubard, F.; et al. A Ladder-like Dopant-free Hole-Transporting Polymer for Hysteresis-less High-Efficiency Perovskite Solar Cells with High Ambient Stability. ChemSusChem 2020, 13, 5058–5066. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, W.; Fu, S.; Chen, L.; Wang, Y.; Xue, Z.; Tao, Y.; Zhang, W.; Song, W.; Fang, J. Achieving over 21% efficiency in inverted perovskite solar cells by fluorinating a dopant-free hole transporting material. J. Mater. Chem. A 2020, 8, 6517–6523. [Google Scholar] [CrossRef]
- Chen, H.-W.; Huang, T.-Y.; Chang, T.-H.; Sanehira, Y.; Kung, C.-W.; Chu, C.-W.; Ikegami, M.; Miyasaka, T.; Ho, K.-C. Efficiency Enhancement of Hybrid Perovskite Solar Cells with MEH-PPV Hole-Transporting Layers. Sci. Rep. 2016, 6, 34319. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Liu, C.; Zhang, J.; Li, X.; Cheng, C.; Tian, Y.; Xu, B.; Liu, C.; Jen, A.K.Y.; Jen, A.K.Y. Intensive Exposure of Functional Rings of a Polymeric Hole-Transporting Material Enables Efficient Perovskite Solar Cells. Adv. Mater. 2018, 30, e1804028. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, L.; Li, Y.; Zhou, X.; She, S.; Wang, X.; Tian, Y.; Jen, A.K.Y.; Xu, B. Highly Stable and Efficient Perovskite Solar Cells with 22.0% Efficiency Based on Inorganic-Organic Dopant-Free Double Hole Transporting Layers. Adv. Funct. Mater. 2020, 30, 1908462. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Wang, X.; Tian, Y.; Jen, A.K.Y.; Xu, B. Side-chain engineering on dopant-free hole-transporting polymers toward highly efficient perovskite solar cells (20.19%). Adv. Funct. Mater. 2019, 29, 1904856. [Google Scholar] [CrossRef]
- Hashimoto, R.; Truong, M.A.; Gopal, A.; Rafieh, A.I.; Nakamura, T.; Murdey, R.; Wakamiya, A. Hole-transporting polymers containing partially oxygen-bridged triphenylamine units and their application for perovskite solar cells. J. Photopolym. Sci. Technol. 2020, 33, 505–516. [Google Scholar] [CrossRef]
- Li, S.; Wan, L.; Chen, L.; Deng, C.; Tao, L.; Lu, Z.; Zhang, W.; Fang, J.; Song, W. Self-Doping a Hole-Transporting Layer Based on a Conjugated Polyelectrolyte Enables Efficient and Stable Inverted Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 11724–11731. [Google Scholar] [CrossRef]
- Oz, S.; Jena, A.K.; Kulkarni, A.; Mouri, K.; Yokoyama, T.; Takei, I.; Unlu, F.; Mathur, S.; Miyasaka, T. Lead(II) Propionate Additive and a Dopant-Free Polymer Hole Transport Material for CsPbI2Br Perovskite Solar Cells. ACS Energy Lett. 2020, 5, 1292–1299. [Google Scholar] [CrossRef]
- Shao, J.-Y.; Yu, B.; Wang, Y.-D.; Lan, Z.-R.; Li, D.; Meng, Q.; Zhong, Y.-W. In-Situ Electropolymerized Polyamines as Dopant-Free Hole-Transporting Materials for Efficient and Stable Inverted Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 5058–5066. [Google Scholar] [CrossRef]
- Park, J.; Yoon, S.E.; Lee, J.; Whang, D.R.; Lee, S.Y.; Shin, S.J.; Han, J.M.; Seo, H.; Park, H.J.; Kim, J.H.; et al. Unraveling Doping Capability of Conjugated Polymers for Strategic Manipulation of Electric Dipole Layer toward Efficient Charge Collection in Perovskite Solar Cells. Adv. Funct. Mater. 2020, 30, 2001560. [Google Scholar] [CrossRef]
- Sun, X.; Deng, X.; Li, Z.; Xiong, B.; Zhong, C.; Zhu, Z.; Li, Z.A.; Jen, A.K.Y. Dopant-Free Crossconjugated Hole-Transporting Polymers for Highly Efficient Perovskite Solar Cells. Adv. Sci. 2020, 7, 1903331. [Google Scholar] [CrossRef]
- Sun, X.; Li, Z.; Yu, X.; Wu, X.; Zhong, C.; Liu, D.; Lei, D.; Jen, A.K.Y.; Li, Z.A.; Zhu, Z. Efficient Inverted Perovskite Solar Cells with Low Voltage Loss Achieved by a Pyridine-Based Dopant-Free Polymer Semiconductor. Angew. Chem. Int. Ed. 2021, 60, 7227–7233. [Google Scholar] [CrossRef]
- Wang, L.; Zhuang, Q.; You, G.; Lin, X.; Li, K.; Lin, Z.; Zhen, H.; Ling, Q. Donor-Acceptor Type Polymers Containing Fused-Ring Units as Dopant-Free, Hole-Transporting Materials for High-Performance Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 12475–12483. [Google Scholar] [CrossRef]
- Li, X.; Tang, X.; Yang, Y.; Ye, T.; Wu, D.; Wang, H.; Li, J.; Wang, X. A dopant-free polymer as hole-transporting material for highly efficient and stable perovskite solar cells. Prog. Photovolt. 2018, 26, 994–1002. [Google Scholar] [CrossRef]
- Putnin, T.; Le, H.; Bui, T.-T.; Jakmunee, J.; Ounnunkad, K.; Peralta, S.; Aubert, P.-H.; Goubard, F.; Farcas, A. Poly(3,4-ethylenedioxythiophene/permethylated β-cyclodextrin) polypseudorotaxane and polyrotaxane: Synthesis, characterization and application as hole transporting materials in perovskite solar cells. Eur. Polym. J. 2018, 105, 250–256. [Google Scholar] [CrossRef]
- Zhu, H.; Johansson, M.B.; Johansson, E.M.J. The Effect of Dopant-Free Hole-Transport Polymers on Charge Generation and Recombination in Cesium-Bismuth-Iodide Solar Cells. ChemSusChem 2018, 11, 1114–1120. [Google Scholar] [CrossRef]
- Miyasaka, T.; Kulkarni, A.; Kim, G.M.; Oez, S.; Jena, A.K. Perovskite Solar Cells: Can We Go Organic-Free, Lead-Free, and Dopant-Free? Adv. Energy Mater. 2020, 10, 1902500. [Google Scholar] [CrossRef]














| HTM | PSK | Cell b | PCE (%) | Std PCE (%) | Voc (mV) | Jsc (mA cm−2) | FF (%) | HOMO (eV) | Bg (eV) | HM (cm2 V−1 S−1) | Stab. (h) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTAA a (commercial) | ((FAPbI3)0.95 (MAPBr3)0.5 | M | 22.6 a | - | 1.119 | 25.0 | 81.7 | - | - | - | >93%, 13 months, NE, (amb. air, rt) | [5] |
| PTAA (synth. published) | MAPbI3 | p | 17.6 | 16.7 c | 1.06 | 22.1 | 75.0 | - | - | - | - | [80] |
| CH3O-PTAA | (FAPbI3)0.85 (MAPbBr3)0.15 | M | 18.79 | 16.34 c | 1.08 | 23.25 | 75.2 | −5.17 | 2.95 | 5.59 × 10−5 | 97%, 1000 h (NE, 85% RH, 85 °C) | [81] |
| P3HT | MAPbI3-xClx | P | 13.6 | - | 0.979 | 24.3 | 57.2 | - | - | - | - | [82] |
| P3HT | CsPbI2Br | P | 15.50 | - | 1.28 | 15.62 | 78.7 | - | - | - | 98%, 30d, (NE, 25% RH, rt) | [83] |
| P3CT-BN | 19.05 | 18.26 d 10.40 | 1.10 | 23.08 | 74.8 | −5.22 | 2.27 | 1.90 × 10−5 | - | [84] | ||
| P3HT (P3CT-BN interlayer) | CsFAMA | P | 19.23 | 19.24 d | 1.10 | 23.52 | 74.2 | - | - | - | 80%, 2300 h (NE, 50% RH, rt) | [85] |
| POWT | FA0.2MA0.8PbI2.9Br0.1 | I | 17.50 | 14.13 e | 1.02 | 24.11 | 71.5 | −5.48 | 2.05 | 5.74 × 10−3 | 85%, 54d, (NE, N2, rt) | [86] |
| PEDOT-C6 | (FAPbI3)0.85 (MAPbBr3)0.15 | M | 12.1 (best) 11.4 (avg) | - | 0.93 | 22.4 | 58.0 | −5.17 | 1.67 | 1.07 × 10−4 | - | [87] |
| PEDOT-C10 | (FAPbI3)0.85 (MAPbBr3)0.15 | M | 16.2 (best) 15.4(avg) | - | 1.06 | 23.3 | 65.0 | −5.17 | 1.66 | 1.24 × 10−4 | 75%, 120h, (NE, 80% RH, rt) | [87] |
| PEDOT-C14 | (FAPbI3)0.85 (MAPbBr3)0.15 | M | 14.8 (best) 13.8 (avg) | - | 10.7 | 20.8 | 67.0 | −5.15 | 1.68 | 0.92 × 10−4 | - | [87] |
| PANI | MAPbI3 | M | 7.34 | - | 0.78 | 14.48 | 65.0 | −5.23 | 2.46 | - | 91%, 1000 h, NE, (amb. air, light, rt) | [88] |
| PT | MAPbI3 | P | 14.70 | 11.0 d | 0.95 | 21.2 | 72.0 | −5.18 | - | - | - | [89] |
| PPN | MAPbI3 | P | 12.80 | 11.0 d | 0.97 | 19.7 | 66.0 | −5.26 | - | - | - | [89] |
| PPP | MAPbI3 | P | 15.80 | 11.0 d | 1.02 | 21.0 | 71.0 | −5.31 | - | - | - | [89] |
| PVCz-OMeDAD | FA0.85 MA0.15Pb(I0.85Br0.15)3 | M | 16.09 | 9.62/18.5 c | 1.085 | 21.96 | 67.5 | −5.24 | 2.75 | 3.44 × 10−4 | - | [90] |
| PVCz-OMeDAD | (3BBA)2.1 MA3.8Pb4.8I11.7Cl3.8 | I | 14.76 | 17.10 c | 1.16 | 16.97 | 75.0 | −5.24 | 2.69 | 1.53 × 10−4 | - | [91] |
| PVCz-OMeTPA | (3BBA)2.1 MA3.8Pb4.8I11.7Cl3.8 | I | 17.22 | 17.10 c | 1.18 | 18.95 | 77.0 | −5.38 | 2.82 | 4.34 × 10−4 | - | [91] |
| P1- PVCzOMeTAD | Cs0.05FA0.81 MA0.14PbI2.55Br0.45 | P | 16.78 | 20.4 c/11.21 | 1.11 | 21.58 | 69.5 | −5.24 | 2.95 | 1.12 × 10−4 | 80%, 30d, (NE, 30% RH, rt) | [92] |
| P2- PVCzOMeTAD | Cs0.05FA0.81 MA0.14PbI2.55Br0.45 | P | 18.45 | 20.4 c/11.21 | 1.13 | 22.34 | 72.7 | −5.21 | 3.19 | 1.32 × 10−4 | 80%, 30d, (NE, 30% RH, rt) | [92] |
| PVP-CZ | Cs0.05(FA0.17MA0.83)0.95Pb(Br0.17I0.83)3 | I | 17.75 (best) 16.61 (avg) | 18.85 c | 0.97 | 22.57 | 81.1 | 4.82 | 2.93 | 3.1 × 10−5 | - | [93] |
| CL1-2 (P1-2) | Cs0.05(FA0.85MA0.15)0.95Pb(I0.9Br0.1)3 | I | 18.7 | 16.0 f | 1.10 | 22.6 | 80.0 | −5.1 | 1.8 | - | 100%, 3000 h, (NE, N2, 85 °C) | [94] |
| DH-MeO-FDPA | MAPbI3-xClx | P | 15.9 (best) 15.50 (avg) | 13.7 d | 1.09 | 19.54 | 74.7 | −5.04 | 2.48 | 5.0 × 10−5 | - | [95] |
| VB-MeO-FDPA | MAPbI3-xClx | P | 18.7 (best) 18.21 (avg) | 13.7 d | 1.15 | 20.89 | 77.8 | −5.18 | 2.27 | 3.0 × 10−4 | 85%, 200 h, (NE, N2, light, rt) | [95] |
| VB-Me-FDPA | MAPbI3-xClx | P | 17.9 (best) 16.50 (avg) | 13.7 d | 1.16 | 20.17 | 76.5 | −5.20 | 2.60 | 1.4 × 10−4 | - | [95] |
| V1162 | Cs0.05(FA0.83MA0.17)0.95Pb(I0.83Br0.17)3 | I | 14.49 | - | 0.88 | 21.60 | 76.4 | −5.26 | - | - | - | [96] |
| V1162 crosslinked | Cs0.05(FA0.83MA0.17)0.95Pb(I0.83Br0.17)3 | I | 14.71 | - | 1.08 | 21.51 | 63.5 | −5.11 | - | - | - | [96] |
| V1187 | Cs0.05(FA0.83MA0.17)0.95Pb(I0.83Br0.17)3 | I | 15.51 | - | 0.93 | 20.73 | 80.3 | −5.26 | - | - | - | [96] |
| V1187 crosslinked | Cs0.05(FA0.83MA0.17)0.95Pb(I0.83Br0.17)3 | I | 16.77 | - | 1.07 | 21.40 | 73.3 | −5.11 | - | - | - | [96] |
| HTM-P1 | Cs0.05FA0.81MA0.14 PbI2.55Br0.45 | M | 16.8 | - | 1.07 | 24.3 | 60.5 | −5.15 | 2.83 | 1.6 × 10−4 | >80%, 30d, NE, (30%, RH rt) | [97] |
| HTM-M1 | Cs0.05FA0.81MA0.14 PbI2.55Br0.45 | M | 9.1 | - | 0.84 | 20.5 | 48.2 | −5.18 | 2.83 | 6.1 × 10−5 | - | [97] |
| HTM | PSK | Cell a | PCE (%) | Std PCE (%) | Voc (mV) | Jsc (mA cm−2) | FF %) | HOMO (eV) | Bg (eV) | HM (cm2 V−1 S−1) | Stab. (h) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCBTDPP | MAPbBr3 | M | 3.04 | 0.76 b | 1.16 | 4.47 | 59.0 | −5.4 | - | 0.02 | - | [77] |
| PCBTDPP | MAPbI3 | M | 5.5 | 6.48 c | 0.83 | 13.86 | 48.0 | −5.4 | - | 0.02 | 5.55%, 900 h, NE, (amb. air, rt) | [77] |
| PDPPT-TT (solv. vap. anneal.) | MA1-yFAyPbI3-xClx | P | 17.8 | 16.5 c | 1.07 | 22.8 | 72.0 | - | - | - | 26%, 72 h, NE, (amb. air, rt) | [100] |
| DPP-P1 | MAPbI3 | M | 7.1 | - | 0.94 | 13.9 | 54.0 | −5.35 | 1.35 | 8.8 × 10−5 | - | [26] |
| DPP-P2 | MAPbI3 | M | 7.3 | - | 0.94 | 11.5 | 67.0 | −5.20 | 1.58 | 4.4 × 10−2 | [26] | |
| DPP-P3 | MAPbI3 | M | 4.5 | - | 0.90 | 9.1 | 55.0 | −5.25 | 1.40 | 2.7 × 10−5 | [26] | |
| DPP-P4 | MAPbI3 | M | 9.1 | - | 0.95 | 13.1 | 74.0 | −5.19 | 1.54 | 3.7 × 10−2 | [26] | |
| DPP-P4 | Cs-Perovskite | M | 14.2 | - | 1.01 | 19.7 | 72.0 | −5.19 | 1.54 | 3.7 × 10−2 | [26] | |
| DPP-P4 | Rb-Perovskite | M | 16.3 | - | 1.05 | 20.6 | 75.0 | −5.19 | 1.54 | 3.7 × 10−2 | 100%, 30d, NE, (dry air, rt) | [26] |
| PDPP3T | MAPbI3 | M | 12.3 | 12.3 c | 0.98 | 20.5 | 61.2 | −5.30 | 1.56 | - | 39.4%, 172 h, NE, (20% RH, rt) | [101] |
| PDPPDBTE (same as PDVT-10) | MAPbI3 | M | 9.2 (doped) | 7.6 c 6.3 b | 0.855 | 14.4 | 74.9 | −5.4 | 1.28 | 0.32 2.77 ann. | 91%, 1000 h, NE, (20% RH, rt) | [102,103] |
| PDVT-10 | MAPbI3 | M | 13.35 (best) 12.97 (avg) | 14.60 d (best) 11.28 (avg) | 0.99 | 20.86 | 65.0 | −5.28 | 1.68 | 8.2 | 100%, 20d, NE, (20% RH, rt), | [104] |
| PBDTP-DTDPP | Cs0.05(MA0.17 FA0.83)0.95Pb(I0.83Br0.17)3 | P | 14.73 | 15.02 c | 1.08 | 19.43 | 68.9 | −5.43 | 1.39 | - | - | [105] |
| P25NH | (MA0.8 FA0.2)Pb(I0.93Cl0.07)3 | P | 18.1 | 15.5 b | 0.998 | 22.18 | 81.9 | −5.18 | 1.68 | 2.1 × 10−2 | 70%, 100h, (NE, 85% RH, rt) | [106] |
| P5NH | (MA0.8 FA0.2)Pb(I0.93Cl0.07)3 | P | 18.1 | 15.5 b | 1.04 | 20.95 | 83.1 | −5.4 | 2.0 | 5.13 × 10−2 | - | [107] |
| 2DP-TPB | FA0.85MA0.15PbI3 | P | 21.53 (best) 20.53 (avg) | 21.98 c | 1.16 | 24.13 | 76.9 | −5.31 | 1.89 | 2.1 × 10−4 | 95%, 1000 h (NE, 30% RH, rt) 81%, 376 h, (NE, N2, 80 °C) | [108] |
| 2DP-TPB | FA0.85MA0.15PbI3 (optimized with 4-fluorobenzamide hydrochloride) | P | 22.17 (optimized perovskite) | 21.98 c | 1.16 | 24.02 | 79.57 | −5.31 | 1.89 | 2.1 × 10−4 | - | [108] |
| HTM | PSK | Cell a | PCE (%) | Std PCE (%) | Voc (mV) | Jsc (mA cm−2) | FF (%) | HOMO (eV) | Bg (eV) | HM (cm2 V−1 s−1) | Cond. (S cm−1) | Stab (h) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTB1 | MAPbI3-xClx | M | 6.0 | 10.9 c | 0.543 | 19.3 | 57.2 | −4.90 | - | 4.5 × 10−4 | - | - | [117] |
| PTB7 | MAPbI3-xClx | M | 7.5 | 10.9 c | 0.699 | 18.0 | 59.8 | −5.15 | - | 5.8 × 10−4 | - | - | [117] |
| PTB7 | MAPbI3 | M | 6.92 (best) 6.30 (avg) | 2.82 b (best) 2.01 (avg) 8.80 c (best) 7.79 (avg) | 0.81 | 15.36 | 56.0 | −5.20 | - | - | - | 20%, 112 h, NE, (55–70% RH, rt) | [98] |
| PTB7 | (QD) α- CsPbI3 | P | 12.55 (best) 11.65 (avg) | 9.82 b (best) 9.30 (avg) 8.20 c (best) 5.20 (avg) | 1.27 | 12.39 | 80.0 | −5.08 | 1.63 | 6.3 × 10−4 | - | - | [122] |
| PTB7-Th | MAPbI3 | M | 3.68 (best) 3.48 (avg) | 2.82 b (best) 2.01 (avg) 8.80 c (best) 7.79 (avg) | 0.87 | 7.54 | 56.0 | −5.12 | - | - | - | Incr. 60%, 112 h, NE, (55–70% RH, rt) | [98] |
| PTB7-Th | (QD) α -CsPbI3 | P | 10.60 | 9.82 b (best) 9.30 (avg) 8.20 c (best) 5.20 (avg) | 1.24 | 11.05 | 78.0 | −5.15 | 1.59 | 1.7 × 10−3 | - | - | [122] |
| PBDT-N0 | (FAPbI3)0.85 (MAPbBr3)0.15 | M | 12.4 (best) 10.6 (avg) | 19.6 c 10.1 | 1.02 | 20.4 | 60.0 | −5.44 | 1.63 | 8.9 × 10−5 | - | 65%, 1400 h, (NE, 85% RH, 80 °C) | [119] |
| PBDT-N5 | (FAPbI3)0.85 (MAPbBr3)0.15 | M | 15.5 (best) 13.2 (avg) | 19.6c 10.1 | 1.05 | 22.4 | 66.0 | −5.45 | 1.52 | 1.2 × 10−4 | - | 75% 1400 h, (NE, 85% RH, 80 °C) | [119] |
| PBDT-N20 | (FAPbI3)0.85 (MAPbBr3)0.15 | M | 18.9 (best) 17.0 (avg) | 19.6 c 10.1 | 1.08 | 23.4 | 75.0 | −5.44 | 1.52 | 4.6 × 10−4 | - | 95% 1400 h, (NE, 85% RH, 80 °C) | [119] |
| RCP-BTT | Cs0.05(FA0.85 MA0.15)0.95Pb(I0.85Br0.15)3 | M | 8.45 | 19.86c | 1.08 | 14.68 | 53.0 | −5.28 | 1.64 | 2.3 × 10−3 | - | >95, 500 h, (NE, 30% RH, rt) 35, 150 h, (E, 85% RH, 85 °C) | [121] |
| PTB-BO | MAPbI3 | M | 7.4 (best) 6.1 (avg) | 7.0 c/11.6 | 0.827 | 14.35 | 62.0 | −5.25 | 1.93 | 5.30 × 10−5 | - | Incr. 21%, 140 h, NE (desiccator, rt) | [123] |
| PTB-DCB21 | MAPbI3 | M | 8.7 (best) 6.0 (avg) | 7.0 c/11.6 | 0.888 | 15.35 | 64.0 | −5.22 | 1.91 | 5.01 × 10−5 | - | Incr. 45%, 140 h, NE (desiccator, rt) | [123] |
| PBDTTT-CP | MAPbI3 | P | 9.95 (best) 9.32 (avg) | 6.17 b (best) 5.47 (avg) | 0.868 | 17.68 | 64.83 | −5.12 | 1.77 | 2.4 × 10−4 | - | - | [124] |
| MAPbI3 | M | 6.64 | 4.24 b | 0.84 | 11.98 | 66.0 | −5.24 | 1.76 | 6.68 × 10−4 | - | - | [125] | |
| RCP | MAPbI3 | P | 17.3 | 3.8/15.3 c | 1.08 | 21.9 | 75.0 | −5.41 | 1.66 | 3.09 × 10−3 | - | 100%, 1400 h, NE, (75% RH, rt) | [126] |
| P1 | MAPbI3 | M | 11.22 | 8.74/18.28 c | 1.03 | 16.12 | 67.68 | −5.05 | 1.60 | 8.84 × 10−3 | - | - | [127] |
| P2 | MAPbI3 | M | 12.29 | 8.74/18.28 c | 1.01 | 16.84 | 72.02 | −5.09 | 1.60 | 1.33 × 10−3 | - | - | [127] |
| P3 | MAPbI3 | M | 17.28 | 8.74/18.28 c | 1.09 | 20.74 | 75.78 | −5.06 | 1.65 | 2.01 × 10−3 | - | 100%, 0d, NE, (amb. air, rt) | [127] |
| asy-PBTBDT | Cs-perovskite | M | 18.3 (best) 17.4 (avg) | 9.9/18.5 c | 1.11 | 22.4 | 73.2 | −5.36 | 1.81 | 1.34 × 10−3 | - | 91%, 30d, NE, (50–75% RH, rt), | [128] |
| PTEG | Cs-perovskite | M | 18.6 | 17.9 c | 1.09 | 22.7 | 75.4 | −5.40 | 1.78 | 1.64 × 10−3 | - | - | [115] |
| PTEG | Cs-perovskite | P | 19.8 | 17.0 c | 1.14 | 22.5 | 77.0 | −5.40 | 1.78 | 1.64 × 10−3 | - | - | [115] |
| Alkoxy-PTEG | Cs0.06FA0.78 MA0.16Pb0.94I2.4Br0.48 | P | 21.2 | 21.0 c | 1.14 | 23.2 | 79.8 | −5.31 | 1.75 | 4.06 × 10−4 | - | 88%, 30d, (NE, 40–50% RH, dark, rt) | [116] |
| pBBTa-BDT1 | MAPbI3 | P | 7.0 (best) 5.05 (avg) | 3.9/15.2 c | 0.77 | 18.1 | 50.6 | −5.11 | 1.39 | 4.6 × 10−5 | 6.6 × 10−6 | 100%, 18 h, NE, (65% RH, 85 °C), 100%, E, (65% RH, 85 °C) | [129] |
| pBBT-BDT2 | MAPbI3 | P | 14.5 (best) 12.05 (avg) | 3.9/15.2 c | 0.95 | 20.3 | 75.2 | −5.21 | 1.37 | 2.0 × 10−3 | 1.3 × 10−5 | 100%, 18 h, NE, (65% RH, 85 °C), 100%, E, (65% RH, 85 °C) | [129] |
| P1-0F | MAPbI3 | M | 9.80 | 8.74/18.47 c | 1.00 | 17.85 | 54.66 | −5.41 | 1.82 | 2.51 × 10−5 | - | >75%, 720 h, NE, (amb. air, rt) | [130] |
| P2-2F | MAPbI3 | M | 14.94 | 8.74/18.47 c | 1.04 | 20.17 | 70.65 | −5.43 | 1.82 | 9.32 × 10−5 | - | >75%, 720 h, NE, (amb. air, rt) | [130] |
| P3-4F | MAPbI3 | M | 10.35 | 8.74/18.47 c | 1.03 | 18.58 | 53.81 | −5.50 | 1.82 | 4.06 × 10−5 | - | >75%, 720 h, NE, (amb. air, rt) | [130] |
| P-TT-TPD | CsFAMA | M | 17.10 (best) (16.96 (avg) | 21.5 c | 1.04 | 21.86 | 74.25 | −5.42 | 1.79 | 1.29 × 10−4 | - | - | [131] |
| K1 | MAPbI3 | P | 16.02 (best) 15.19 (avg) | - | 1.06 | 19.22 | 78.0 | −5.29 | 1.96 | 2.96 × 10−5 | - | 85%, 200 h, NE, (amb. air, rt) | [132] |
| K2 | MAPbI3 | P | 3.85 (best) 2.9 (avg) | - | 0.92 | 7.69 | 55.0 | −5.35 | 1.48 | 2.55 × 10−7 | - | - | [132] |
| K3 | MAPbI3 | P | 12.50 (best) 11.98 (avg) | - | 1.02 | 16.94 | 73.0 | −5.40 | 1.97 | 1.41 × 10−6 | - | - | [132] |
| K4 | MAPbI3 | P | 6.45 (best) 5.84 (avg) | - | 0.94 | 11.07 | 63.0 | −5.31 | 1.53 | 9.67 × 10−7 | - | [132] | |
| K5 | MAPbI3 | P | 14.65 (best) 13.83 (avg) | - | 1.05 | 18.32 | 76.0 | −5.40 | 1.92 | 5.68 × 10−6 | - | - | [132] |
| K6 | MAPbI3 | P | 13.77 (bes) 13.06 (avg) | - | 1.04 | 17.75 | 75.0 | −5.42 | 1.92 | 4.89 × 10−6 | - | - | [132] |
| R1 | MAPbI3 | M | 15.88 | -- | 0.98 | 23.70 | 68.3 | −5.42 | 1.60 | 3.7 × 10−3 | - | 100%, 500 h, NE (75% RH, rt) | [133] |
| R2 | MAPbI3 | M | 13.57 | - | 0.88 | 23.10 | 66.2 | −5.37 | 1.60 | 5.8 × 10−4 | around 67%, 500 h, NE (75% RH, rt) | [133] | |
| PBT1-C | CsFAMA | M | 19.06 (best) 18.6 (avg) | 14.96 b (best) 14.53 (avg) | 1.05 | 22.37 | 81.22 | −5.43 | 1.93 | 1.5 × 10−3 | - | 87%, 300 h NE (inert gas, rt) | [134] |
| PBDB-T | CsPbI2Br | P | 14.20 (best) 13.56 (avg) | 1.12 | 15.33 | 81.7 | −5.28 | 1.80 | 3.07 × 10−4 | - | - | [135] | |
| PBDB-T-2Cl | CsPbI2Br | P | 14.03 (best) 13.21 (avg) | 1.21 | 14.19 | 77.2 | −5.51 | 1.80 | 4.56 × 10−4 | - | - | [135] | |
| PBDB-T-2F | CsPbI2Br | P | 14.87 (best) 14.12 (avg) | 1.20 | 14.62 | 81.7 | −5.47 | 1.80 | 5.68 × 10−4 | - | - | [135] | |
| PBDB-T-Si | CsPbI2Br | P | 15.60 (best) 14.95 (avg) | 1.20 | 15.28 | 82.5 | −5.45 | 1.87 | 6.75 × 10−4 | - | - | [135] | |
| P1-B | MAPbI3 | M | 14.9 (best) 13.3 (avg) | 20.4 c (best) 19.7 (avg) | 0.96 | 21.5 | 63.8 | −5.16 | 2.27 | 3.0 × 10−5 | - | - | [136] |
| P2-T | MAPbI3 | M | 19.6 (best) 19.2 (avg) | 20.4 c (best) 19.7 (avg) | 1.07 | 22.8 | 77.6 | −5.27 | 2.03 | 3.8 × 10−4 | - | 96%, 40d, NE (20–70% RH, rt) | [136] |
| P3-T-F | MAPbI3 | M | 20.3 (best) 19.7 (avg) | 20.4 c (best) 19.7 (avg) | 1.09 | 22.8 | 78.5 | −5.38 | 1.98 | 5.7 × 10−4 | - | 96%, 40d, NE (20–70% RH, rt) | [136] |
| P1-Th-BDT-BO | (FAPbI3)0.85 (MAPbBr3)0.15 | P | 18.30 (best) 17.76 (avg) | 19.35 c (best) 18.56 (avg) | 1.06 | 22.39 | 75.0 | −5.43 | 2.09 | 1.72 × 10−3 | - | >89%, 96 h, NE (80% RH, rt) | [137] |
| P2-OR-BDT-BO | (FAPbI3)0.85 (MAPbBr3)0.15 | P | 7.57 (best) 7.55 (avg) | 19.35 c (best) 18.56 (avg) | 0.81 | 21.75 | 42.9 | −5.76 | 2.20 | 3.78 × 10−4 | - | >89%, 96 h, NE (80% RH, rt) | [137] |
| P3-OR-BDT-T | (FAPbI3)0.85 (MAPbBr3)0.15 | P | 13.03 (best) 12.92 (avg) | 19.35 c (best) 18.56 (avg) | 0.93 | 22.03 | 63.3 | −5.30 | 2.13 | 2.54 × 10−4 | - | >89%, 96 h, NE (80% RH, rt) | [137] |
| PBDT[2F]T | MAPbI3 | P | 17.52 (best) 16.55 (avg) | 20.20 c 15.59 d | 1.06 | 22.64 | 72.60 | −5.29 | 2.1 | 9.2 × 10−6 | - | 91%, 16d, NE (30% RH, 30 °C) | [138] |
| PBDT(T)[2F]T | MAPbI3 | P | 11.63 (best) 10.25 (avg) | 20.20 c 15.59 d | 1.03 | 16.59 | 67.95 | −5.22 | 2.0 | - | - | - | [138] |
| PBDT[2H]T | MAPbI3 | P | 14.24 (best) 13.50 (avg) | 20.20 c 15.59 d | 1.05 | 20.89 | 64.97 | −5.03 | 2.1 | 2.2 × 10−6 [139] | - | - | [138] |
| HTM | PSK | Cell a | PCE (%) | Std PCE (%) b | Voc (mV) | Jsc (mA cm−2) | FF (%) | HOMO eV) | Bg (eV) | HM (cm2 V−1 S−1) | Stab. (h) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCDTBT | MAPbI3 | P | 16.5 | - | 1.05 | 21.9 | 72.0 | −5.3 | - | - | - | [99] |
| PCDTBT | MAPbI3 | P | 17.1 (best) 16.43 (avg) | 19.4 b (best) 18.81 (avg) | 1.05 | 21.62 | 72.34 | −5.35 | - | - | >90%, 30d, E, (RH 40–70%, 25-35 °C), | [150] |
| PCDTBT1 | MAPbI3 | P | 19.1 (best) 18.67 (avg) | 19.4 b (best) 18.81 (avg) | 1.10 | 21.78 | 77.63 | −5.38 | - | - | >90%, 30d, E, (RH 40–70%, 25–35 °C), | [150] |
| PCDTBT8 | MAPbI3 | P | 15.4 (best) 14.81 (avg) | 19.4 b (best) 18.81 (avg) | 1.02 | 21.29 | 67.31 | −5.40 | - | - | 25%, 30d, E, (RH 40–70%, 25–35 °C), | [150] |
| BF-NMe3 | MAPbI3 | I | 14.92 (best) 14.65 (avg) | 12.81 PEDOT: PSS | 1.01 | 18.51 | 79.7 | −5.40 | 1.88 | - | >90% 960 h, (NE, 25% RH, rt) | [152] |
| BF SO3 | MAPbI3 | I | 17.14 (best) 16.85 (avg) | 12.81 PEDOT: PSS | 1.05 | 19.92 | 82.1 | −5.40 | 1.88 | - | >90% 960 h, (NE, 25% RH, rt) | [152] |
| BF-NH3 | MAPbI3 | I | 17.71 (best) 16.94 (avg) | 12.81 PEDOT: PSS | 1.05 | 20.10 | 84.04 | −5.39 | 1.86 | - | ->90% 960 h, (NE, 25% RH, rt) | [152] |
| TTB-TTQ | MAPbI3-xClx | M | 6,2 | 11.5 c (best) 10.1 (avg) | 0.94 | 11.8 | 55.4 | −5.11 | 1.61 | 1.01 × 10−4 | - | [153] |
| BTTP | MAPbI3 | M | 3.80 | 7.55 c/12.53 c | 0.78 | 10.11 | 48.2 | −5.40 | 2.2 | - | - | [154] |
| BTTP-CN | MAPbI3 | M | 3.48 | 7.55 c/12.53 c | 0.69 | 10.01 | 50.3 | −5.25 | 2.13 | - | - | [154] |
| PVBT-SO3 | MAPbI3 | I | 18.4 (best) 17.57 (avg) | - | 1.06 | 22.5 | 77.0 | −5.27 | 1.72 | - | - | [155] |
| PVF2BT-SO3 | MAPbI3 | I | 13.6 (best) 13.2 (avg) | - | 1.05 | 18.89 | 69.0 | −5.40 | 1.78 | - | - | [155] |
| PVF4-SO3 | MAPbI3 | I | 7.3 (best) 6.69 (avg) | - | 0.99 | 12.9 | 56.0 | −5.66 | 2.16 | - | - | [155] |
| PDTIDTBT | CsFAMA | M | 17.9 | 18.63 (spiro) | 1.09 | 22.5 | 79.0 | −5.35 | 2.06 | 5.24 × 10−4 | [156] | |
| PDTIDTBT | CsFAMA (PMMA) | M | 19.89 | 19.28 (spiro) | 1.14 | 22.6 | 77.2 | −5.35 | 2.06 | 5.24 × 10−4 | >93%, 42d, (NE, 42% RH, rt) | [156] |
| DTS | MAPbI3(Cl) | I | 17.27 | 16.92 c | 1.01 | 21.97 | 77.46 | −5.01 | 1.67 | 8.83 × 10−5 | - | [157] |
| DTS | MAPbI3(Cl)/ (PSBMA) | I | 20.17 | 18.02 c | 1.10 | 22.44 | 81.67 | −5.01 | 1.67 | 8.83 × 10−5 | >83%, 400 h (NE, N2, 85 °C) >94%, 1500 h (NE, 20% RH, rt) | [157] |
| CDT | MAPbI3(Cl) | I | 17.02 | 16.92 c | 0.98 | 22.23 | 78.17 | −5.14 | 1.54 | 8.43 × 10−5 | - | [157] |
| CDT | MAPbI3(Cl)/ (PSBMA) | I | 20.05 | 18.02 c | 1.08 | 22.75 | 81.59 | −5.14 | 1.54 | 8.43 × 10−5 | >83%, 400 h (NE, N2, 85 °C) >94%, 1500 h (NE, 20% RH, rt) | [157] |
| DTP | MAPbI3(Cl) | I | 14.87 | 16.92 | 1.10 | 21.44 | 63.05 | −5.23 | 1.45 | 4.96 × 10−5 | - | [157] |
| DTP | MAPbI3(Cl)/ (PSBMA) | I | 17.60 | 18.02 c | 1.04 | 21.59 | 78.38 | −5.23 | 1.45 | 4.96 × 10−5 | >83%, 400 h (NE, N2, 85 °C) >94%, 1500 h (NE, 20% RH, rt) | [157] |
| 3,6PCzTPA | (FAPbI3)0.85 (MAPbBr3)0.15 | I | 16.4 | 18.1 c | 0.96 | 21.93 | 77.9 | −5.18 | 3.18 | 1.46 × 10−5 | - | [158] |
| 2,7PCzTPA | (FAPbI3)0.85 (MAPbBr3)0.15 | I | 17.1 | 18.1 c | 1.07 | 21.78 | 73.2 | −5.23 | 2.86 | 1.49 × 10−6 | - | [158] |
| 3,6-2,7-PCzTPA | (FAPbI3)0.85 (MAPbBr3)0.15 | I | 18.4 | 18.1 c | 1.04 | 22.66 | 77.9 | −5.22 | 3.00 | 1.74 × 10−5 | - | [158] |
| PC1 | MAPbI3 | M | 8.8 | 20.3 spiro | 0.85 | 21.3 | 49.1 | −5.37 | 2.37 | 4.2 × 10−5 | - | [159] |
| PC2 | MAPbI3 | M | 18.3 | 20.3 spiro | 1.06 | 21.7 | 76.2 | −5.17 | 2.13 | 7.3 × 10−4 | - | [159] |
| PC3 | MAPbI3 | M | 20.8 | 20.3 spiro | 1.11 | 23.5 | 80.0 | −5.16 | 2.09 | 8.1 × 10−4 | 100%, 1000 h (NE, light, rt) | [159] |
| PDTPC-1 | CsFAMA | P | 16.96 | - | 1.08 | 21.94 | 71.58 | −5.11 | 1.60 | 3.98 × 10−3 | [160] | |
| PDTPC-2 | CsFAMA | P | 12.45 | - | 0.97 | 20.15 | 63.70 | −4.97 | 1.57 | 2.94 × 10−3 | [160] |
| HTM | PSK | Cell a | PCE (%) | Std PCE (%)b | Voc (mV) | Jsc (mA cm−2) | FF (%) | HOMO (eV) | Bg (eV) | HM (cm2 V−1 S−1) | Stab. (h) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PF8-TAA | MAPbBr3 | M | 6.0 | 5.9 d | 1.36 | 6.3 | 70.0 | −5.44 | 0.02 | - | [162] | |
| PF8-TAA | MAPbI3 | M | 4.6 | 16.2 d | 0.92 | 8.9 | 56.0 | −5.44 | 0.02 | - | [162] | |
| PIF8-TAA | MAPbBr3 | M | 6.7 | 5.9 d | 1.40 | 6.1 | 79.0 | −5.51 | 0.04 | - | [162] | |
| PIF8-TAA | MAPbI3 | M | 9.1 | 16.2 d | 1.04 | 19.0 | 46.0 | −5.51 | 0.04 | - | [162] | |
| PSQ1 | α- CsPbI2Br | P | 13.6 | 14.4 c | 1.24 | 14.4 | 76.1 | −5.21 | 1.79 | 1.09 × 10−3 | - | [163] |
| PSQ2 | α- CsPbI2Br | P | 15.5 | 14.4 c | 1.27 | 15.4 | 79.0 | −5.31 | 1.86 | 9.87 × 10−3 | 83%, 300 h, (light rt) | [163] |
| P3-3,5-diOMe | α- CsPbI2Br | P | 11.6 | 14.4 c | 1.30 | 12.9 | 69.4 | −5.27 | 2.47 | 1.45 × 10−5 | - | [163] |
| PBDTT | Cs0.05FA0.81MA0.14 PbI2.55Br0.45 | P | 20.28 | 13.88 b 18.32 c | 1.12 | 23.64 | 76.67 | −5.31 | 1.87 | 7.10 × 10−4 | 80%, 720 h, NE (30% RH, rt) | [165] |
| PBTTT | Cs0.05FA0.81MA0.14 PbI2.55Br0.45 | P | 19.48 | 13.88 b 18.32 c | 1.10 | 22.30 | 79.57 | −5.24 | 1.89 | 6.99 × 10−4 | 80%, 720 h, NE (30% RH, rt) | [165] |
| IDTB | Cs0.05FA0.81MA0.14 PbI2.55Br0.4 | P | 19.38 (best) 16.39 (avg) | 18.22 b | 1.107 | 23.06 | 75.9 | −5.20 | 2.19 | 2.9 × 10−5 | 70%, 60d (NE, 30–40% RH, 30 °C) | [166] |
| PFDT-COOH | FA1-xMAxPbI3 | I | 20.64 (best) 19.65 (avg) | - | 1.079 | 24.78 | 77.2 | −5.22 | 2.18 | - | 80%, 720 h, NE (N2, 85 °C) | [167] |
| PFDT-2F-COOH | FA1-xMAxPbI3 | I | 21.68 (best) 20.51 (avg) | - | 1.111 | 24.86 | 78.5 | −5.32 | 2.3 | - | 80%, 720 h, NE (N2, 85 °C) | [167] |
| MEH-PPV | MAPbI3 | M | 9.65 | 6.74 b/13.38 c | 0.88 | 17.36 | 63.0 | −5.40 | 2.1 | [168] | ||
| DTB | FA0.85MA0.15 PbI2.55Br0.45 | M | 19.68 (best) 19.19 (avg) 19.5 (cert) | 19.81 c (best) 19.38 (avg) | 1.10 | 25.50 | 69.85 | −4.96 | 2.14 | 1.0 × 10−4 | - | [169] |
| DTB/CuSCN | FAPbI3)0.92 (MAPbBr3)0.08 | P | 22.0 | - | 1.15 | 24.31 | 78.6 | − | - | 0.014 | 90%, 1000 h, (NE, 60% RH, rt) | [170] |
| DTB(0%DEG) | Cs0.05FA0.81MA0.14 PbI2.55Br0.45 | M | 19.65 (best) 18.57 (avg) | - | 1.10 | 25.01 | 71.48 | −4.99 | 2.14 | 1.74 × 10−4 | 87%, NE (amb.air, rt) | [171] |
| DTB(3%DEG) | Cs0.05FA0.81MA0.14 PbI2.55Br0.45 | M | 20.19 (best) 19.22 (avg) 20.10 (cert) | - | 1.14 | 23.64 | 75.08 | −4.97 | 2.14 | 3.22 × 10−4 | 92%, NE (amb.air, rt) | [171] |
| DTB(10%DEG) | Cs0.05FA0.81MA0.14 PbI2.55Br0.45 | M | 17.82 (best) 16.78 (avg) | - | 1.05 | 24.02 | 72.42 | −4.94 | 2.14 | 2.02 × 10−4 | 87%, NE (amb.air, rt) | [171] |
| PTA-P1 | MAPbI3 | M | 12.1 (best) 10.3 (avg) | 17.6 d, 4.9 | 1.04 | 21.6 | 54.0 | −5.07 | 2.58 | 2.8 × 10−−4 | [172] | |
| PTA-P2 | MAPbI3 | M | 1.3 (best) 0.97 (avg) | 17.6 d 4.9 | 0.83 | 11.3 | 14.0 | −5.44 | 2.84 | 1.5 × 10−−5 | [172] | |
| PTA-P3 | MAPbI3 | M | 9.1 (best) 8.2 (avg) | 17.6 d 4.9 | 0.97 | 20.6 | 45.0 | −5.16 | 2.64 | 3.4 × 10−−5 | [172] | |
| PTA-P4 | MAPbI3 | M | 11.1 (best) 10.3 (avg) | 17.6 d 4.9 | 1.04 | 21.6 | 50.0 | −5.21 | 2.26 | 2.6 × 10−−4 | [172] | |
| PTPADT-SO3Na | MAPbI3 | I | 18.85 | 13.86 e | 1.08 | 21.26 | 79.2 | −5.18 | 2.37 | - | 81%, 500 h (NE, 50% RH, rt) 79%, 200 h (NE, 50% RH, 80 °C) | [173] |
| Poly (DTSTPD-r- BThTPD) | CsPbI2Br:1%Pb (CH3CH2COO)2 | M | 14.58 | 11.14 b | 1.15 | 15.66 | 81.0 | −5.44 | 1.70 | 1.5 × 10−3 | - | [174] |
| poly-TATPyr | (FAPbI3)x (MAPbBr3)(1-x) | I | 16.5 (best) 15.5 (avg) | 16.3 d | 0.998 | 23.3 | 71.0 | −5.4 | - | 1.0 × 10−5 | 91%, 1000 h (NE, dark, rt) | [175] |
| PIDF-BT | 17.45 | [176] | ||||||||||
| PPE1 | (FAPbI3)0.83 (MAPbBr3)0.17 | I | 11.13 (best) 9.50 (avg) | 21.56 d | 1.03 | 15.53 | 70.0 | −5.08 | 2.45 | 2.2 × 10−6 | - | [177] |
| PPE2 | (FAPbI3)0.83 (MAPbBr3)0.17 | I | 19.33 (best) 18.10(avg) | 21.56 d | 1.07 | 22.84 | 79 | −5.06 | 2.70 | 1.9 × 10−6 | - | [177] |
| PPE2 (PSK passivation with PEAI) | (FAPbI3)0.83(MAPbBr3)0.17 | I | 21.31 best 19.77 (avg) | 21.56 d | 1.18 | 22.30 | 81 | −5.06 | 2.70 | 1.9 × 10−6 | - | [177] |
| PPY1 | (FA0.92MA0.08)0.9Cs0.1Pb(I0.92Br0.08)3 | I | 15.08 | 20.98 d | 0.92 | 22.15 | 74.0 | −4.96 | 2.67 | 1.30 × 10−3 | - | [178] |
| PPY2 | (FA0.92MA0.08)0.9Cs0.1Pb(I0.92Br0.08)3 | I | 22.41 | 20.98 d | 1.16 | 23.56 | 82.0 | −5.17 | 2.48 | 1.90 × 10−3 | >97%, 500h (NE, light, rt9 | [178] |
| PDT-T | P | 18.69 | 13.69 b | 1.13 | 21.36 | 77.63 | −5.45 | 1.88 | 6.29 × 10−4 | >90%, 100d, (E, 30% RH, rt) | [179] | |
| PDTT-T | P | 19.02 | 13.69 b | 1.14 | 21.68 | 76.79 | −5.44 | 1.89 | 7.41 × 10−4 | >90%, 100d, (E, 30% RH, rt) | [179] | |
| TZ-P1 | FA0.81MA0.15PbI2.5Br0.45 | M | 10.26 | 13.02 c | 0.94 | 17.62 | 62.0 | −5.21 | 1.88 | - | - | [180] |
| TZ-P2 | FA0.81MA0.15PbI2.5Br0.45 | M | 13.21 | 13.02 c | 1.02 | 19.87 | 65.0 | −5.19 | 1.83 | - | 80%, 113d, NE, (N2, rt) | [180] |
| TZ-P3 | FA0.81MA0.15PbI2.5Br0.45 | M | 8.47 | 13.02 c | 0.90 | 16.47 | 57.0 | −5.20 | 1.84 | - | - | [180] |
| PEDOT-TMbCD-P1 | MAPbI3 | M | 5.54 (best) 3.64 (avg) | 6.27 c/14.41 c | 0.74 | 19.51 | 38.0 | −4.20 | 2.60 | - | - | [181] |
| PEDOT-TMbCD-P2 | MAPbI3 | M | 3.80 (best) 2.02 (avg) | 6.27 c/14.41 c | 0.60 | 13.55 | 47.0 | −4.32 | 2.20 | - | - | [181] |
| TQ1 | CsBi3I10 | M | 0.51 | 0.36 b/0.003 c | 0.48 | 2.9 | 37.0 | −5.70 | 1.78 | 1.6 × 10−3 | [182] | |
| P3TI | CsBi3I10 | M | 0.47 | 0.36 b/0.003 c | 0.47 | 2.6 | 38.0 | −5.69 | 1.72 | 1.8 × 10−3 | [182] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desoky, M.M.H.; Bonomo, M.; Barbero, N.; Viscardi, G.; Barolo, C.; Quagliotto, P. Polymeric Dopant-Free Hole Transporting Materials for Perovskite Solar Cells: Structures and Concepts towards Better Performances. Polymers 2021, 13, 1652. https://doi.org/10.3390/polym13101652
Desoky MMH, Bonomo M, Barbero N, Viscardi G, Barolo C, Quagliotto P. Polymeric Dopant-Free Hole Transporting Materials for Perovskite Solar Cells: Structures and Concepts towards Better Performances. Polymers. 2021; 13(10):1652. https://doi.org/10.3390/polym13101652
Chicago/Turabian StyleDesoky, Mohamed M. H., Matteo Bonomo, Nadia Barbero, Guido Viscardi, Claudia Barolo, and Pierluigi Quagliotto. 2021. "Polymeric Dopant-Free Hole Transporting Materials for Perovskite Solar Cells: Structures and Concepts towards Better Performances" Polymers 13, no. 10: 1652. https://doi.org/10.3390/polym13101652