Macromolecular Engineering of Poly(catechol) Cathodes towards High-Performance Aqueous Zinc-Polymer Batteries
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Design of Zn||Poly(catechol) Aqueous Battery
3.2. Comparative Electrochemical Performance of Zn||P(4VC)100 vs. Zn||P(4VC86-stat-SS14) Full-Cells
3.2.1. Comparative Electrochemical Performance at Room Temperature
3.2.2. Comparative Electrochemical Performance at Low Temperatures
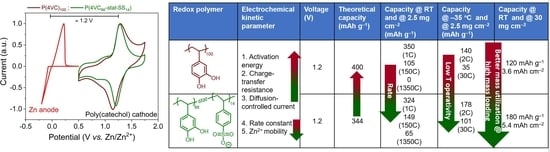
3.3. Electrochemical Kinetic Evaluation of Redox Reactions
3.4. Development of More Practical Batteries Using High Mass Loading Electrodes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Larcher, D.; Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Bondarev, D.; Singhal, V.; Yushin, G. Ten years left to redesign lithium-ion batteries. Nature 2018, 559, 467–470. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Park, K.-Y.; Kim, H.; Kim, S.-W.; Kang, K. Aqueous Rechargeable Li and Na Ion Batteries. Chem. Rev. 2014, 114, 11788–11827. [Google Scholar] [CrossRef]
- Chao, D.; Zhou, W.; Xie, F.; Ye, C.; Li, H.; Jaroniec, M.; Qiao, S.-Z. Roadmap for advanced aqueous batteries: From design of materials to applications. Sci. Adv. 2020, 6, eaba4098. [Google Scholar] [CrossRef]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent Advances in Zn-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Konarov, A.; Voronina, N.; Jo, J.H.; Bakenov, Z.; Sun, Y.-K.; Myung, S.-T. Present and Future Perspective on Electrode Materials for Rechargeable Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2620–2640. [Google Scholar] [CrossRef]
- Du, W.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C.C. Challenges in the material and structural design of zinc anode towards high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 2020, 13, 3330–3360. [Google Scholar] [CrossRef]
- Cui, J.; Guo, Z.; Yi, J.; Liu, X.; Wu, K.; Liang, P.; Li, Q.; Liu, Y.; Wang, Y.; Xia, Y.; et al. Organic Cathode Materials for Rechargeable Zinc Batteries: Mechanisms, Challenges, and Perspectives. ChemSusChem 2020, 13, 2160–2185. [Google Scholar] [CrossRef]
- Ming, J.; Guo, J.; Xia, C.; Wang, W.; Alshareef, H.N. Zinc-ion batteries: Materials, mechanisms, and applications. Mater. Sci. Eng. R Rep. 2019, 135, 58–84. [Google Scholar] [CrossRef]
- Zeng, X.; Hao, J.; Wang, Z.; Mao, J.; Guo, Z. Recent progress and perspectives on aqueous Zn-based rechargeable batteries with mild aqueous electrolytes. Energy Storage Mater. 2019, 20, 410–437. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Han, C.; Wang, Z.; Liu, Z.; Tang, Z.; Zhi, C. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Ma, L.; Schroeder, M.A.; Borodin, O.; Pollard, T.P.; Ding, M.S.; Wang, C.; Xu, K. Realizing high zinc reversibility in rechargeable batteries. Nat. Energy 2020, 5, 743–749. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Pan, A.; Liang, S. Recent Advances in Aqueous Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Chen, L.; An, Q.; Mai, L. Recent Advances and Prospects of Cathode Materials for Rechargeable Aqueous Zinc-Ion Batteries. Adv. Mater. Interfaces 2019, 6, 1900387. [Google Scholar] [CrossRef]
- Xu, S.; Sun, M.; Wang, Q.; Wang, C. Recent progress in organic electrodes for zinc-ion batteries. J. Semicond. 2020, 41, 091704. [Google Scholar] [CrossRef]
- Kang, L.; Cui, M.; Zhang, Z.; Jiang, F. Rechargeable Aqueous Zinc-Ion Batteries with Mild Electrolytes: A Comprehensive Review. Batter. Supercaps 2020, 3, 966–1005. [Google Scholar] [CrossRef]
- Muench, S.; Wild, A.; Friebe, C.; Häupler, B.; Janoschka, T.; Schubert, U.S. Polymer-Based Organic Batteries. Chem. Rev. 2016, 116, 9438–9484. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Ariga, K. Redox-Active Polymers for Energy Storage Nanoarchitectonics. Joule 2017, 1, 739–768. [Google Scholar] [CrossRef] [Green Version]
- Esser, B.; Dolhem, F.; Becuwe, M.; Poizot, P.; Vlad, A.; Brandell, D. A perspective on organic electrode materials and technologies for next generation batteries. J. Power Sources 2021, 482, 228814. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, G.; Ku, K.; Yoon, K.; Jung, S.-K.; Lim, H.-D.; Kang, K. Recent Progress in Organic Electrodes for Li and Na Rechargeable Batteries. Adv. Mater. 2018, 30, 1704682. [Google Scholar] [CrossRef]
- Amin, K.; Mao, L.; Wei, Z. Recent Progress in Polymeric Carbonyl-Based Electrode Materials for Lithium and Sodium Ion Batteries. Macromol. Rapid Commun. 2019, 40, 1800565. [Google Scholar] [CrossRef] [PubMed]
- Poizot, P.; Gaubicher, J.; Renault, S.; Dubois, L.; Liang, Y.; Yao, Y. Opportunities and Challenges for Organic Electrodes in Electrochemical Energy Storage. Chem. Rev. 2020, 120, 6490–6557. [Google Scholar] [CrossRef]
- Chen, Y.; Zhuo, S.; Li, Z.; Wang, C. Redox polymers for rechargeable metal-ion batteries. EnergyChem 2020, 100030. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J. Prospects of organic electrode materials for practical lithium batteries. Nat. Rev. Chem. 2020, 4, 127–142. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Q. Recent Progress in Multivalent Metal (Mg, Zn, Ca, and Al) and Metal-Ion Rechargeable Batteries with Organic Materials as Promising Electrodes. Small 2019, 15, 1805061. [Google Scholar] [CrossRef]
- McAllister, B.T.; Kyne, L.T.; Schon, T.B.; Seferos, D.S. Potential for Disruption with Organic Magnesium-Ion Batteries. Joule 2019, 3, 620–624. [Google Scholar] [CrossRef] [Green Version]
- Hager, M.D.; Esser, B.; Feng, X.; Schuhmann, W.; Theato, P.; Schubert, U.S. Polymer-Based Batteries—Flexible and Thin Energy Storage Systems. Adv. Mater. 2020, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.; Aqil, A.; Ouhib, F.; Admassie, S.; Inganäs, O.; Jérôme, C.; Detrembleur, C. Bioinspired Redox-Active Catechol-Bearing Polymers as Ultrarobust Organic Cathodes for Lithium Storage. Adv. Mater. 2017, 29, 1703373. [Google Scholar] [CrossRef]
- Patil, N.; Cordella, D.; Aqil, A.; Debuigne, A.; Admassie, S.; Jérôme, C.; Detrembleur, C. Surface- and Redox-Active Multifunctional Polyphenol-Derived Poly(ionic liquid)s: Controlled Synthesis and Characterization. Macromolecules 2016, 49, 7676–7691. [Google Scholar] [CrossRef]
- Patil, N.; Aqil, M.; Aqil, A.; Ouhib, F.; Marcilla, R.; Minoia, A.; Lazzaroni, R.; Jérôme, C.; Detrembleur, C. Integration of Redox-Active Catechol Pendants into Poly(ionic liquid) for the Design of High-Performance Lithium-Ion Battery Cathodes. Chem. Mater. 2018, 30, 5831–5835. [Google Scholar] [CrossRef]
- Hernández, G.; Casado, N.; Zamarayeva, A.M.; Duey, J.K.; Armand, M.; Arias, A.C.; Mecerreyes, D. Perylene Polyimide-Polyether Anodes for Aqueous All-Organic Polymer Batteries. ACS Appl. Energy Mater. 2018, 1, 7199–7205. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Sarang, K.; Lutkenhaus, J.; Verduzco, R. Side-Chain Engineering for High-Performance Conjugated Polymer Batteries. Adv. Funct. Mater. 2021, 31, 2009263. [Google Scholar] [CrossRef]
- Aqil, M.; Ouhib, F.; Aqil, A.; El Idrissi, A.; Detrembleur, C.; Jérôme, C. Polymer ionic liquid bearing radicals as an active material for organic batteries with ultrafast charge-discharge rate. Eur. Polym. J. 2018, 106, 242–248. [Google Scholar] [CrossRef]
- Chhin, D.; Padilla-Sampson, L.; Malenfant, J.; Rigaut, V.; Nazemi, A.; Schougaard, S.B. Conducting Polymers Doped with Bifunctional Copolymers for Improved Organic Batteries. ACS Appl. Energy Mater. 2019, 2, 7781–7790. [Google Scholar] [CrossRef]
- Patil, N.; Mavrandonakis, A.; Jérôme, C.; Detrembleur, C.; Palma, J.; Marcilla, R. Polymers Bearing Catechol Pendants as Universal Hosts for Aqueous Rechargeable H+, Li-Ion, and Post-Li-ion (Mono-, Di-, and Trivalent) Batteries. ACS Appl. Energy Mater. 2019, 2, 3035–3041. [Google Scholar] [CrossRef]
- Dawut, G.; Lu, Y.; Miao, L.; Chen, J. High-performance rechargeable aqueous Zn-ion batteries with a poly(benzoquinonyl sulfide) cathode. Inorg. Chem. Front. 2018, 5, 1391–1396. [Google Scholar] [CrossRef]
- Yue, X.; Liu, H.; Liu, P. Polymer grafted on carbon nanotubes as a flexible cathode for aqueous zinc ion batteries. Chem. Commun. 2019, 55, 1647–1650. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, W.; Li, H.; Xu, Q. Cross-Conjugated Polycatechol Organic Cathode for Aqueous Zinc-Ion Storage. ChemSusChem 2020, 13, 188–195. [Google Scholar] [CrossRef]
- Nam, K.W.; Park, S.S.; dos Reis, R.; Dravid, V.P.; Kim, H.; Mirkin, C.A.; Stoddart, J.F. Conductive 2D metal-organic framework for high-performance cathodes in aqueous rechargeable zinc batteries. Nat. Commun. 2019, 10, 4948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häupler, B.; Rössel, C.; Schwenke, A.M.; Winsberg, J.; Schmidt, D.; Wild, A.; Schubert, U.S. Aqueous zinc-organic polymer battery with a high rate performance and long lifetime. NPG Asia Mater. 2016, 8, e283. [Google Scholar] [CrossRef]
- Li, P.; Fang, Z.; Zhang, Y.; Mo, C.; Hu, X.; Jian, J.; Wang, S.; Yu, D. A high-performance, highly bendable quasi-solid-state zinc–organic battery enabled by intelligent proton-self-buffering copolymer cathodes. J. Mater. Chem. A 2019, 7, 17292–17298. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, F.; Liu, L.; Lei, K.; Hou, X.; Xu, G.; Meng, H.; Shi, J.; Li, F. A High-Power Aqueous Zinc–Organic Radical Battery with Tunable Operating Voltage Triggered by Selected Anions. ChemSusChem 2020, 13, 2239–2244. [Google Scholar] [CrossRef]
- Yu, M.; Chandrasekhar, N.; Raghupathy, R.K.M.; Ly, K.H.; Zhang, H.; Dmitrieva, E.; Liang, C.; Lu, X.; Kühne, T.D.; Mirhosseini, H.; et al. A High-Rate Two-Dimensional Polyarylimide Covalent Organic Framework Anode for Aqueous Zn-Ion Energy Storage Devices. J. Am. Chem. Soc. 2020, 142, 19570–19578. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, T.; Cheng, J.; Guan, Q.; Wang, B. Bioinspired Interface Design of Sewable, Weavable, and Washable Fiber Zinc Batteries for Wearable Power Textiles. Adv. Funct. Mater. 2020, 30, 2004430. [Google Scholar] [CrossRef]
- Khayum, M.A.; Ghosh, M.; Vijayakumar, V.; Halder, A.; Nurhuda, M.; Kumar, S.; Addicoat, M.; Kurungot, S.; Banerjee, R. Zinc ion interactions in a two-dimensional covalent organic framework based aqueous zinc ion battery. Chem. Sci. 2019, 10, 8889–8894. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Zhao, X.; Xu, Y.; Zhang, Y.; Yang, Y.; Hu, A.; Tang, Q.; Song, X.; Jiang, C.; Chen, X. Sewable and Cuttable Flexible Zinc-Ion Hybrid Supercapacitor Using a Polydopamine/Carbon Cloth-Based Cathode. ACS Sustain. Chem. Eng. 2020, 8, 16028–16036. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Y.; Lu, Y.; Li, L.; Wan, F.; Zhang, K.; Chen, J. Modulating electrolyte structure for ultralow temperature aqueous zinc batteries. Nat. Commun. 2020, 11, 4463. [Google Scholar] [CrossRef]
- Sun, T.; Li, Z.; Zhi, Y.; Huang, Y.; Fan, H.J.; Zhang, Q. Poly(2,5-Dihydroxy-1,4-Benzoquinonyl Sulfide) as an Efficient Cathode for High-Performance Aqueous Zinc–Organic Batteries. Adv. Funct. Mater. 2021, 2010049. [Google Scholar] [CrossRef]
- Patil, N.; de la Cruz, C.; Ciurduc, D.; Mavrandonakis, A.; Palma, J.; Marcilla, R. An Ultrahigh Performance Zinc-Organic Battery Using Poly(catechol) Cathode in Zn(TFSI)2 Based Concentrated Aqueous Electrolytes. Adv. Energy Mater. 2021. [Google Scholar] [CrossRef]
- Molina, A.; Patil, N.; Ventosa, E.; Liras, M.; Palma, J.; Marcilla, R. Electrode Engineering of Redox-Active Conjugated Microporous Polymers for Ultra-High Areal Capacity Organic Batteries. ACS Energy Lett. 2020, 5, 2945–2953. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Sterby, M.; Emanuelsson, R.; Mamedov, F.; Strømme, M.; Sjödin, M. Investigating electron transport in a PEDOT/Quinone conducting redox polymer with in situ methods. Electrochim. Acta 2019, 308, 277–284. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.-L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.; Mavrandonakis, A.; Jérôme, C.; Detrembleur, C.; Casado, N.; Mecerreyes, D.; Palma, J.; Marcilla, R. High-performance all-organic aqueous batteries based on a poly(imide) anode and poly(catechol) cathode. J. Mater. Chem. A 2021, 9, 505–514. [Google Scholar] [CrossRef]
- Park, S.; Kristanto, I.; Jung, G.Y.; Ahn, D.B.; Jeong, K.; Kwak, S.K.; Lee, S.-Y. A single-ion conducting covalent organic framework for aqueous rechargeable Zn-ion batteries. Chem. Sci. 2020, 11, 11692–11698. [Google Scholar] [CrossRef]
- Mo, F.; Chen, Z.; Liang, G.; Wang, D.; Zhao, Y.; Li, H.; Dong, B.; Zhi, C. Zwitterionic Sulfobetaine Hydrogel Electrolyte Building Separated Positive/Negative Ion Migration Channels for Aqueous Zn-MnO 2 Batteries with Superior Rate Capabilities. Adv. Energy Mater. 2020, 10, 2000035. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, N.; Palma, J.; Marcilla, R. Macromolecular Engineering of Poly(catechol) Cathodes towards High-Performance Aqueous Zinc-Polymer Batteries. Polymers 2021, 13, 1673. https://doi.org/10.3390/polym13111673
Patil N, Palma J, Marcilla R. Macromolecular Engineering of Poly(catechol) Cathodes towards High-Performance Aqueous Zinc-Polymer Batteries. Polymers. 2021; 13(11):1673. https://doi.org/10.3390/polym13111673
Chicago/Turabian StylePatil, Nagaraj, Jesus Palma, and Rebeca Marcilla. 2021. "Macromolecular Engineering of Poly(catechol) Cathodes towards High-Performance Aqueous Zinc-Polymer Batteries" Polymers 13, no. 11: 1673. https://doi.org/10.3390/polym13111673