Micro-Oxygenation in Upflow Anaerobic Sludge Bed (UASB) Reactors Using a Silicon Membrane for Sulfide Oxidation
Abstract
:1. Introduction
2. Materials and Methods
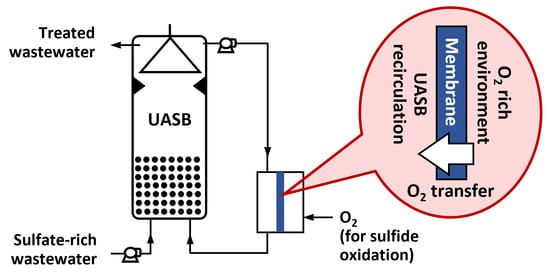
2.1. Reactor Setup
2.2. Reactor Operation
2.3. Mass Balances
3. Results
3.1. Reactor Performance
3.2. Sulfur Balance
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pokorna-Krayzelova, L.; Bartacek, J.; Kolesarova, N.; Jenicek, P. Microaeration for hydrogen sulfide removal in UASB reactor. Bioresour. Technol. 2014, 172, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Cirne, D.G.; Van Der Zee, F.P.; Fernandez-Polanco, M.; Fernandez-Polanco, F. Control of sulphide during anaerobic treatment of S-containing wastewaters by adding limited amounts of oxygen or nitrate. Rev. Environ. Sci. Bio/Technol. 2008, 7, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Sabumon, P.C. Development of enhanced sulphidogenesis process for the treatment of wastewater having low COD/SO42− ratio. J. Hazard. Mater. 2008, 159, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Liamleam, W.; Annachhatre, A.P. Electron donors for biological sulfate reduction. Biotechnol. Adv. 2007, 25, 452–463. [Google Scholar] [CrossRef]
- Wang, Z.; Banks, C.J. Report: Anaerobic digestion of a sulphate-rich high-strength landfill leachate: The effect of differential dosing with FeCl3. Waste Manag. Res. 2006, 24, 289–293. [Google Scholar] [CrossRef]
- Jeong, T.-Y.; Cha, G.-C.; Seo, Y.-C.; Jeon, C.; Choi, S.S. Effect of COD/sulfate ratios on batch anaerobic digestion using waste activated sludge. J. Ind. Eng. Chem. 2008, 14, 693–697. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Chen, J.L.; Ortiz, R.; Steele, T.W.; Stuckey, D. Toxicants inhibiting anaerobic digestion: A review. Biotechnol. Adv. 2014, 32, 1523–1534. [Google Scholar] [CrossRef]
- Dawoud, U.; Vanweele, S.; Szklarska-Smialowska, Z. The effect of H2S on the crevice corrosion of aisi 410 and ca6nm stainless steels in 3.5% nacl solutions. Corros. Sci. 1992, 33, 295–306. [Google Scholar] [CrossRef]
- Benatti, C.T.; Tavares, C.R.G.; Lenzi, E. Sulfate removal from waste chemicals by precipitation. J. Environ. Manag. 2009, 90, 504–511. [Google Scholar] [CrossRef]
- Ranade, D. Evaluation of the use of sodium molybdate to inhibit sulphate reduction during anaerobic digestion of distillery waste. Bioresour. Technol. 1999, 68, 287–291. [Google Scholar] [CrossRef]
- Isa, M.H.; Anderson, G. Molybdate inhibition of sulphate reduction in two-phase anaerobic digestion. Process. Biochem. 2005, 40, 2079–2089. [Google Scholar] [CrossRef]
- Speece, R.E. Anaerobic biotechnology for industrial wastewater treatment. Environ. Sci. Technol. 1983, 17, 416A–427A. [Google Scholar] [CrossRef] [PubMed]
- Krayzelova, L.; Bartacek, J.; Diaz, I.; Jeison, D.; Volcke, E.I.; Jenicek, P. Microaeration for hydrogen sulfide removal during anaerobic treatment: A review. Rev. Environ. Sci. Bio/Technol. 2015, 14, 703–725. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; Tsapekos, P.; Alfaro, N.; Díaz, I.; Fdz-Polanco, M.; Rafiee, S.; Angelidaki, I. A review on prospects and challenges of biological H2S removal from biogas with focus on biotrickling filtration and microaerobic desulfurization. Biofuel Res. J. 2017, 4, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Van Der Zee, F.; Villaverde, S.; García, P.; Fdz-Polanco, F. Sulfide removal by moderate oxygenation of anaerobic sludge environments. Bioresour. Technol. 2007, 98, 518–524. [Google Scholar] [CrossRef]
- Muñoz, R.; Meier, L.; Diaz, I.; Jeison, D. A review on the state-of-the-art of physical/chemical and biological technologies for biogas upgrading. Rev. Environ. Sci. Bio/Technol. 2015, 14, 727–759. [Google Scholar] [CrossRef] [Green Version]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017; ISBN 9780875532875. [Google Scholar]
- Camiloti, P.R.; Valdes, F.; Delforno, T.; Bartacek, J.; Zaiat, M.; Jeison, D. A membrane aerated biofilm reactor for sulfide control from anaerobically treated wastewater. Environ. Technol. 2018, 40, 2354–2363. [Google Scholar] [CrossRef]
- Pokorna-Krayzelova, L.; Bartacek, J.; Vejmelkova, D.; Alvarez, A.A.; Slukova, P.; Prochazka, J.; Volcke, E.I.; Jenicek, P. The use of a silicone-based biomembrane for microaerobic H2S removal from biogas. Sep. Purif. Technol. 2017, 189, 145–152. [Google Scholar] [CrossRef]
- Schwarz, A.; Suárez, J.I.; Aybar, M.; Nancucheo, I.; Martínez, P.; Rittmann, B.E. A membrane-biofilm system for sulfate conversion to elemental sulfur in mining-influenced waters. Sci. Total. Environ. 2020, 740, 140088. [Google Scholar] [CrossRef]
- Sahinkaya, E.; Hasar, H.; Kaksonen, A.H.; Rittmann, B.E. Performance of a Sulfide-Oxidizing, Sulfur-Producing Membrane Biofilm Reactor Treating Sulfide-Containing Bioreactor Effluent. Environ. Sci. Technol. 2011, 45, 4080–4087. [Google Scholar] [CrossRef]
- Pokorna-Krayzelova, L.; Bartacek, J.; Theuri, S.N.; Gonzalez, C.A.S.; Prochazka, J.; Volcke, E.I.; Jenicek, P. Microaeration through a biomembrane for biogas desulfurization: Lab-scale and pilot-scale experiences. Environ. Sci. Water Res. Technol. 2018, 4, 1190–1200. [Google Scholar] [CrossRef]
- Valdes, F.; Muñoz, E.; Chamy, R.; Ruiz, G.; Vergara, C.; Jeison, D.; Ruiz-Filippi, G. Effect of sulphate concentration and sulphide desorption on the combined removal of organic matter and sulphate from wastewaters using expanded granular sludge bed (EGSB) reactors. Electron. J. Biotechnol. 2006, 9. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.; Khanal, S.K. A little breath of fresh air into an anaerobic system: How microaeration facilitates anaerobic digestion process. Biotechnol. Adv. 2018, 36, 1971–1983. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Imai, T.; Ukita, M.; Li, F.; Yuasa, A. Effect of limited aeration on the anaerobic treatment of evaporator condensate from a sulfite pulp mill. Chemosphere 2007, 66, 924–929. [Google Scholar] [CrossRef]
- Boopathy, R.; Bokang, H.; Daniels, L. Biotransformation of furfural and 5-hydroxymethyl furfural by enteric bacteria. J. Ind. Microbiol. Biotechnol. 1993, 11, 147–150. [Google Scholar] [CrossRef]
- Balk, M.; Altinbas, M.; Rijpstra, W.I.C.; Damsté, J.S.S.; Stams, A.J.M. Desulfatirhabdium butyrativorans gen. nov., sp. nov., a butyrate-oxidizing, sulfate-reducing bacterium isolated from an anaerobic bioreactor. Int. J. Syst. Evol. Microbiol. 2008, 58, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, D.; Ueki, A.; Shizuku, T.; Ohtaki, Y.; Ueki, K. Desulfovibrio butyratiphilus sp. nov., a Gram-negative, butyrate-oxidizing, sulfate-reducing bacterium isolated from an anaerobic municipal sewage sludge digester. Int. J. Syst. Evol. Microbiol. 2010, 60, 595–602. [Google Scholar] [CrossRef] [Green Version]





| Parameter | Method | Periodicity |
|---|---|---|
| Chemical oxygen demand (COD) | Standard methods [18] | 2–3 times/week |
| Biogas volumetric production | Water displacement | 2–3 times/week |
| Gas and biogas composition (CH4/CO2/N2/O2) | Gas chromatography with TCD detector (Clarus 500, Perkin Elmer, Waltham, MA, USA) | 2–3 times/week |
| Sulfate | Ionic chromatography (Compact IC plus 882, Methrom, Herisau, Switzerland) | 2–3 times/week |
| Thiosulfate | Ionic chromatography (Compact IC plus 882, Methrom, Herisau, Switzerland) | 2–3 times/week |
| Dissolved sulfide | Spectrophotometry (sulfide reagent set, methylene blue, product number 2244500, Hach, Loveland, CO, USA) | 2–3 times/week |
| Biogas sulfide | Gas chromatography with FPD detector (Clarus 500, Perkin Elmer, Waltham, MA, USA) | 2–3 times/week |
| Parameter | Units | Days 1–10 (No Micro-Oxygenation) | Days 16–66 (with Micro-Oxygenation) |
|---|---|---|---|
| Sulfide leaving the system in the gas phase | (g-S L−1 d−1) | 0.0122 | 0.0067 |
| Sulfide leaving the system in the liquid phase | (g-S L−1 d−1) | 0.0585 | 0.0253 |
| Sulfide leaving the system (Total) | (g-S L−1 d−1) | 0.0707 | 0.032 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés, F.; Camiloti, P.R.; Bartacek, J.; Torres-Aravena, Á.; Toledo-Alarcón, J.; Zaiat, M.; Jeison, D. Micro-Oxygenation in Upflow Anaerobic Sludge Bed (UASB) Reactors Using a Silicon Membrane for Sulfide Oxidation. Polymers 2020, 12, 1990. https://doi.org/10.3390/polym12091990
Valdés F, Camiloti PR, Bartacek J, Torres-Aravena Á, Toledo-Alarcón J, Zaiat M, Jeison D. Micro-Oxygenation in Upflow Anaerobic Sludge Bed (UASB) Reactors Using a Silicon Membrane for Sulfide Oxidation. Polymers. 2020; 12(9):1990. https://doi.org/10.3390/polym12091990
Chicago/Turabian StyleValdés, Freddy, Priscila Rosseto Camiloti, Jan Bartacek, Álvaro Torres-Aravena, Javiera Toledo-Alarcón, Marcelo Zaiat, and David Jeison. 2020. "Micro-Oxygenation in Upflow Anaerobic Sludge Bed (UASB) Reactors Using a Silicon Membrane for Sulfide Oxidation" Polymers 12, no. 9: 1990. https://doi.org/10.3390/polym12091990