Chitosan-ZnO Nanocomposites Assessed by Dielectric, Mechanical, and Piezoelectric Properties
Abstract
:1. Introduction
2. Materials and Methods
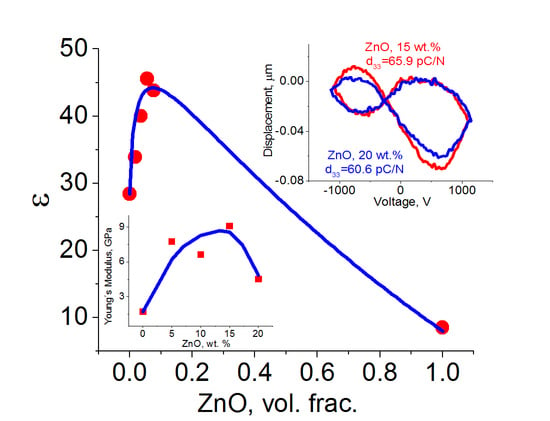
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalpana, V.N.; Rajeswari, V.D. A Review on Green Synthesis, Biomedical Applications, and Toxicity Studies of ZnO NPs. Bioinorg. Chem. Appl. 2018, 2018, 3569758. [Google Scholar] [CrossRef] [PubMed]
- Mishra, Y.K.; Adelung, R. ZnO tetrapod materials for functional applications. Mater. Today 2018, 21, 631–651. [Google Scholar] [CrossRef]
- Klingshirn, C. ZnO: Material, Physics and Applications. Chem. Phys. Chem. 2007, 8, 782–803. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, T.; Perumal, V.; Khor, S.F.; Anthony, L.S.; Gopinath, S.C.B.; Mohamed, N.M. The recent development of polysaccharides biomaterials and their performance for supercapacitor applications. Mater. Res. Bull. 2020, 126, 110839. [Google Scholar] [CrossRef]
- Jin, C.; Hao, N.; Xu, Z.; Trase, I.; Nie, Y.; Dong, L.; Closson, A.; Chen, Z.; Zhang, J.X.J. Flexible piezoelectric nanogenerators using metal-doped ZnO-PVDF films. Sens. Actuators A Phys. 2020, 305, 111912. [Google Scholar] [CrossRef]
- Fakhri, P.; Amini, B.; Bagherzadeh, R.; Kashfi, M.; Latifi, M.; Yavari, N.; Kani, S.A.; Kong, L. Flexible hybrid structure piezoelectric nanogenerator based on ZnO nanorod/PVDF nanofibers with improved output. RSC Adv. 2019, 9, 10117. [Google Scholar] [CrossRef] [Green Version]
- Sinar, D.; Knopf, G.K. Disposable piezoelectric vibration sensors with PDMS/ZnO transducers on printed graphene-cellulose electrodes. Sens. Actuators A Phys. 2020, 302, 111800. [Google Scholar] [CrossRef]
- Buzarovska, A.; Dinescu, S.; Lazar, A.D.; Serban, M.; Pircalabioru, G.G.; Costache, M.; Gualandi, C.; Averous, L. Nanocomposite foams based on flexible biobased thermoplastic polyurethane and ZnO nanoparticles as potential wound dressing materials. Mater. Sci. Eng. C 2019, 104, 109893. [Google Scholar] [CrossRef]
- Choudhary, S. Structural, optical, dielectric and electrical properties of (PEO–PVP)–ZnO nanocomposites. J. Phys. Chem. Solids 2018, 121, 196–209. [Google Scholar] [CrossRef]
- Carvalho, J.T.; Dubceac, V.; Grey, P.; Cunha, I.; Fortunato, E.; Martins, R.; Clausner, A.; Zschech, E.; Pereira, L. Fully Printed Zinc Oxide Electrolyte-Gated Transistors on Paper. Nanomaterials 2019, 9, 169. [Google Scholar] [CrossRef] [Green Version]
- Souza, V.G.L.; Rodrigues, C.; Valente, S.; Pimenta, C.; Pires, J.R.A.; Alves, M.M.; Santos, C.F.I.; Coelhoso, M.; Fernando, A.L. Eco-Friendly ZnO/Chitosan Bionanocomposites Films for Packaging of Fresh Poultry Meat. Coatings 2020, 10, 110. [Google Scholar] [CrossRef] [Green Version]
- Ridwan, R.; Rihayat, T.; Suryani, S.; Ismi, A.S.; Nurhanifa, N.; Riskina, S. Combination of poly lactid acid zinc oxide nanocomposite for antimicrobial packaging application. IOP Conf. Ser. Mater. Sci. Eng. 2020, 830, 042018. [Google Scholar] [CrossRef]
- Rodrigues, C.; De Mello, J.M.M.; Dalcanton, F.; Macuvele, D.L.P.; Padoin, N.; Fiori, M.A.; Soares, C.; Riella, H.G. Mechanical, Thermal and Antimicrobial Properties of Chitosan-Based-Nanocomposite with Potential Applications for Food Packaging. J. Polym. Environ. 2020, 28, 1216–1236. [Google Scholar] [CrossRef]
- Vaseeharan, B.; Sivakamavalli, J.; Thaya, R. Synthesis and characterization of chitosan-ZnO composite and its antibiofilm activity against aquatic bacteria. J. Compos. Mater. 2013, 49, 177–184. [Google Scholar] [CrossRef]
- Li, L.-H.; Deng, J.-C.; Deng, H.-R.; Liu, Z.-L.; Xin, L. Synthesis and characterization of chitosan/ZnO nanoparticle composite membranes. Carbohydr. Res. 2010, 345, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Anandhavelu, S.; Dhanasekaran, V.; Sethuraman, V.; Park, H.J. Chitin and Chitosan Based Hybrid Nanocomposites for Super Capacitor Applications. J. Nanosci. Nanotechnol. 2017, 17, 1321–1328. [Google Scholar] [CrossRef]
- Qiu, B.; Xu, X.-F.; Deng, R.-H.; Xia, G.-Q.; Shang, X.-F.; Zhou, P.-H. Construction of chitosan/ZnO nanocomposite film by in situ precipitation. Int. J. Biol. Macromol. 2019, 122, 82–87. [Google Scholar] [CrossRef]
- Abarna, B.; Preethi, T.; Rajarajeswari, G.R. Single-pot solid-state synthesis of ZnO/chitosan composite for photocatalytic and antitumour applications. J. Mater. Sci. Mater. Electron. 2019, 30, 21355–21368. [Google Scholar] [CrossRef]
- Rahman, M.; Muraleedaran, P.K.; Mujeeb, V.M.A. Applications of chitosan powder with in situ synthesized nano ZnO particles as an antimicrobial agent. Int. J. Biol. Macromol. 2015, 77, 266–272. [Google Scholar] [CrossRef]
- Jayasuriya, A.C.; Aryaei, A.; Jayatissa, A.H. ZnO nanoparticles induced effects on nanomechanical behavior and cell viability of chitosan films. Mater. Sci. Eng. C 2013, 33, 3688–3696. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Maiti, S.; Liu, D. Morphological, Mechanical and Thermal Study of ZnO Nanoparticle Reinforced Chitosan Based Transparent Biocomposite Films. J. Inst. Eng. (India) Ser. D 2014, 95, 35–41. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Pechyen, C. Physico-Chemical Properties of ZnO and Chitosan Composite for Packaging Material. J. Biobased Mater. Bioenergy 2017, 11, 183–192. [Google Scholar] [CrossRef]
- Rahman, P.M.; Mujeeb, V.M.A.; Muraleedharan, K.; Thomas, S.K. Chitosan/nano ZnO composite films: Enhanced mechanical, antimicrobial and dielectric properties. Arab. J. Chem. 2018, 11, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Indumathi, M.P.; Sarojini, K.S.; Rajarajeswari, G.R. Antimicrobial and biodegradable chitosan/cellulose acetate phthalate/ZnO nano composite films with optimal oxygen permeability and hydrophobicity for extending the shelf life of black grape fruits. Int. J. Biol. Macromol. 2019, 132, 1112–1120. [Google Scholar] [CrossRef]
- Hezma, A.M.; Rajeh, A.; Mannaa, M.A. An insight into the effect of zinc oxide nanoparticles on the structural, thermal, mechanical properties and antimicrobial activity of Cs/PVA composite. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123821. [Google Scholar] [CrossRef]
- Alturki, A.M. Effect of Preparation Method on the Particles Size, Dielectric Constant and Antibacterial Properties of ZnO Nanoparticles and Thin Film of ZnO/Chitosan. Orient. J. Chem. 2018, 34, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Warlimont, H.; Martienssen, W. (Eds.) Springer Handbook of Materials Data; Springer Nature: Cham, Switzerland, 2018. [Google Scholar]
- Swaroop, K.; Naveen, C.S.; Jayanna, H.S.; Somashekarappa, H.M. Effect of gamma irradiation on DC electrical conductivity of ZnO nanoparticles. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2015; Volume 1665, p. 050100. [Google Scholar] [CrossRef]
- Naveen, C.S.; Jayanna, H.S.; Lamani, A.R.; Rajeeva, M.P. Temperature dependent DC electrical conductivity studies of ZnO nanoparticle thick films prepared by simple solution combustion method. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2014. [Google Scholar]
- Wang, R.S.; An, J.; Ong, H. Studies of Interfacial Optical and Electrical Properties on Transparent Dielectrics/ZnO Systems; Cambridge University Press (CUP): Cambridge, UK, 2006; Volume 928, pp. 35–78. [Google Scholar]
- Wang, G.-S.; Wu, Y.-Y.; Zhang, X.; Li, Y.; Guo, L.; Cao, M. Controllable synthesis of uniform ZnO nanorods and their enhanced dielectric and absorption properties. J. Mater. Chem. A 2014, 2, 8644–8651. [Google Scholar] [CrossRef]
- Wang, G.; Deng, Y.; Xiang, Y.; Guo, L. Fabrication of radial ZnO nanowire clusters and radial ZnO/PVDF composites with enhanced dielectric properties. Adv. Funct. Mater. 2008, 18, 2584–2592. [Google Scholar] [CrossRef]
- Rajesh, K.; Crasta, V.; Kumar, N.B.R.; Shetty, G. Effect of ZnO nanofiller on dielectric and mechanical properties of PVA/PVP blend. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; Volume 2162, p. 020096. [Google Scholar]
- Wang, G.; Deng, Y.; Guo, L. Single-Crystalline ZnO Nanowire Bundles: Synthesis, Mechanism and Their Application in Dielectric Composites. Chem. A Eur. J. 2010, 16, 10220–10225. [Google Scholar] [CrossRef]
- Hemalatha, K.S.; Rukmani, K. Concentration dependent dielectric, AC conductivity and sensing study of ZnO-polyvinyl alcohol nanocomposite films. Int. J. Nanotechnol. 2017, 14, 961–974. [Google Scholar] [CrossRef]
- Efros, A.L.; Shklovskii, B.I. Critical Behaviour of Conductivity and Dielectric Constant near the Metal-Non-Metal Transition Threshold. Phys. Status Solidi (B) 1976, 76, 475–485. [Google Scholar] [CrossRef]
- Bergman, D.J.; Stroud, D. Physical Properties of Macroscopically Inhomogeneous Media. Methods Exp. Phys. 1992, 46, 147–269. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. ZnO-Reinforced Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Bionanocomposites with Antimicrobial Function for Food Packaging. ACS Appl. Mater. Interfaces 2014, 6, 9822–9834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dıez-Pascual, A.M.; Xu, C.; Luque, R. Development and characterization of novel poly(ether ether ketone)/ZnO bionanocomposites. J. Mater. Chem. B 2014, 2, 3065. [Google Scholar] [CrossRef] [Green Version]
- Poddar, M.K.; Sharma, S.; Moholkar, V.S. Investigations in two-step ultrasonic synthesis of PMMA/ZnO nanocomposites by in–situ emulsion polymerization. Polymer 2016, 99, 453–469. [Google Scholar] [CrossRef]
- Pantani, R.; Gorrasi, G.; Vigliotta, G.; Murariu, M.; Dubois, P. PLA-ZnO nanocomposite films: Water vapor barrier properties and specific end-use characteristics. Eur. Polym. J. 2013, 49, 3471–3482. [Google Scholar] [CrossRef]
- Abbas, M.; Buntinx, M.; Deferme, W.; Peeters, R. (Bio)polymer/ZnO Nanocomposites for Packaging Applications: A Review of Gas Barrier and Mechanical Properties. Nanomaterials 2019, 9, 1494. [Google Scholar] [CrossRef] [Green Version]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. High-Performance Aminated Poly(phenylene sulfide)/ZnO Nanocomposites for Medical Applications. ACS Appl. Mater. Interfaces 2014, 6, 10132–10145. [Google Scholar] [CrossRef] [Green Version]
- AboMostafa, H.M.; El Komy, G.M. Enhancement of Structural, Dielectric and Mechanical Properties of Ps: Fe Doped ZnO Based Polymer Nanocomposites. J. Inorg. Organomet. Polym. Mater. 2019, 29, 908–916. [Google Scholar] [CrossRef]
- Kumar-Krishnan, S.; Prokhorov, E.; Ramirez-Cardona, M.; Hernández-Landaverde, M.A.; Zárate-Triviño, D.G.; Kovalenko, Y.; Sanchez, I.C.; Mendez-Nonell, J.; Bárcenas, G.L. Novel gigahertz frequency dielectric relaxations in chitosan films. Soft Matter 2014, 10, 8673–8684. [Google Scholar] [CrossRef]
- Abdelhady, M.M. Preparation and Characterization of Chitosan/Zinc Oxide Nanoparticles for Imparting Antimicrobial and UV Protection to Cotton Fabric. Int. J. Carbohydr. Chem. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- González-Campos, J.; Prokhorov, E.; Bárcenas, G.; Sanchez, I.C.; Lara-Romero, J.; Mendoza-Duarte, M.E.; Villaseñor, F.; Guevara-Olvera, L. Chitosan/silver nanoparticles composite: Molecular relaxations investigation by dynamic mechanical analysis and impedance spectroscopy. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 739–748. [Google Scholar] [CrossRef]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Manuspiya, H. A comparative study of dielectric and ferroelectric properties of sol–gel-derived BaTiO3 bulk ceramics with fine and coarse grains. Appl. Phys. A 2018, 124, 713. [Google Scholar] [CrossRef]
- Prokhorov, E.; Luna-Bárcenas, G.; González-Campos, J.B.; Kovalenko, Y.; García-Carvajal, Z.Y.; Mota-Morales, J. Proton conductivity and relaxation properties of chitosan-acetate films. Electrochim. Acta 2016, 215, 600–608. [Google Scholar] [CrossRef]
- Vyshatko, N.P.; Brioso, P.M.; De La Cruz, J.P.; Vilarinho, P.M.; Kholkin, A.L. Fiber-optic based method for the measurements of electric-field induced displacements in ferroelectric materials. Rev. Sci. Instrum. 2005, 76, 85101. [Google Scholar] [CrossRef]
- Fialka, J. Determination of the Piezoelectric Charge Constant D33 Measured by the Laser Interferometer and Frequency Method. In Annals of DAAAM for 2010 & Proceedings of the 21st International DAAAM Symposium; DAAAM International: Vienna, Austria, 2010; Volume 21. [Google Scholar]
- Sinha, N.; Ray, G.; Bhandari, S.; Godara, S.; Kumar, B. Synthesis and enhanced properties of cerium doped ZnO nanorods. Ceram. Int. 2014, 40, 12337–12342. [Google Scholar] [CrossRef]
- Thakur, P.; Kool, A.; Hoque, N.A.; Bagchi, B.; Khatun, F.; Biswas, P.; Brahma, D.; Roy, S.; Banerjee, S.; Das, S. Superior performances of in situ synthesized ZnO/PVDF thin film based self-poled piezoelectric nanogenerator and self-charged photo-power bank with high durability. Nano Energy 2018, 44, 456–467. [Google Scholar] [CrossRef]
- Satthiyaraju, M.; Ramesh, T. Nanomechanical, Mechanical Responses and Characterization of Piezoelectric Nanoparticle-Modified Electrospun PVDF Nanofibrous Films. Arab. J. Sci. Eng. 2019, 44, 5697–5709. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, S.; Kadlec, A. Piezoelectric and dielectric properties of nanoporous polyvinylidence fluoride (PVDF) films. Behav. Mech. Multifunct. Mater. Compos. 2016 2016, 9800, 98000. [Google Scholar] [CrossRef]
- Singh, H.H.; Khare, N. Flexible ZnO-PVDF/PTFE based piezo-tribo hybrid nanogenerator. Nano Energy 2018, 51, 216–222. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Z.; Fang, L.; Guo, S.; Erturun, U.; Zhu, Z.; West, J.E.; Ghosh, S.; Kang, S.H. Analytical, numerical, and experimental studies of viscoelastic effects on the performance of soft piezoelectric nanocomposites. Nanoscale 2017, 9, 14215–14228. [Google Scholar] [CrossRef]
- Godavarti, U.; Mote, V.; Dasari, M. Role of cobalt doping on the electrical conductivity of ZnO nanoparticles. J. Asian Ceram. Soc. 2017, 5, 391–396. [Google Scholar] [CrossRef]
- Barber, P.; Balasubramanian, S.; Anguchamy, Y.; Gong, S.; Wibowo, A.; Gao, H.; Ploehn, H.J.; Loye, H.-C. Polymer Composite and Nanocomposite Dielectric Materials for Pulse Power Energy Storage. Materials 2009, 2, 1697–1733. [Google Scholar] [CrossRef]
- Kirkpatrick, S. Percolation and Conduction. Rev. Mod. Phys. 1973, 45, 574–588. [Google Scholar] [CrossRef]
- Cai, W.-Z.; Tu, S.-T.; Gong, J.-M. A Physically Based Percolation Model of the Effective Electrical Conductivity of Particle Filled Composites. J. Compos. Mater. 2006, 40, 2131–2142. [Google Scholar] [CrossRef]
- Vo, H.T.; Shi, F.G. Towards model-based engineering of optoelectronic packaging materials: Dielectric constant modeling. Microelectron. J. 2002, 33, 409–415. [Google Scholar] [CrossRef]
- Todd, M.G.; Shi, F.G. Characterizing the interphase dielectric constant of polymer composite materials: Effect of chemical coupling agents. J. Appl. Phys. 2003, 94, 4551. [Google Scholar] [CrossRef]
- Campbell, S.L.; Chancelier, J.P.; Nikoukhah, R. Modeling and Simulation in Scilab/Scicos with ScicosLab 4.4; Springer: New York, NY, USA, 2010. [Google Scholar]
- Zakaria, A.Z.; Shelesh-Nezhad, K. The effects of interphase and interface haracteristics on the tensile behaviour of POM/CaCO3 nanocomposites. Nanomater. Nanotechnol. 2004, 17, 1–10. [Google Scholar]
- Nunthanid, J.; Laungtana-Anan, M.; Sriamornsak, P.; Limmatvapirat, S.; Puttipipatkhachorn, S.; Lim, L.-Y.; Khor, E. Characterization of chitosan acetate as a binder for sustained release tablets. J. Control. Release 2004, 99, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Mezina, E.A.; Lipatova, I.M.; Losev, N.V. Effect of mechanical activation on rheological and film-forming properties of suspensions of barium sulfate in chitosan solutions. Russ. J. Appl. Chem. 2011, 84, 486–490. [Google Scholar] [CrossRef]
- Aguirre-Loredo, R.Y.; Hernández, A.I.R.; Morales-Sánchez, E.; Gómez-Aldapa, C.A.; Velazquez, G. Effect of equilibrium moisture content on barrier, mechanical and thermal properties of chitosan films. Food Chem. 2016, 196, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Vázquez, M.; Velazquez, G. Environmentally Friendly Films Combining Bacterial Cellulose, Chitosan, and Polyvinyl Alcohol: Effect of Water Activity on Barrier, Mechanical, and Optical Properties. Biomacromolecules 2019, 21, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Baxter, S.C.; Burrows, B.J.; Fralick, B.S. Mechanical percolation in nanocomposites: Microstructure and micromechanics. Probabilistic Eng. Mech. 2016, 44, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Padmanabhan, V. Percolation of high-density polymer regions in nanocomposites: The underlying property for mechanical reinforcement. J. Chem. Phys. 2013, 139, 144904. [Google Scholar] [CrossRef]
- Fralick, B.S.; Gatzke, E.P.; Baxter, S.C. Three-dimensional evolution of mechanical percolation in nanocomposites with random microstructures. Probabilistic Eng. Mech. 2012, 30, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hua, Z.; Shi, X.; Chen, Y. Preparation, Structure, and Property of Highly Filled Polyamide 11/BaTiO3 Piezoelectric Composites Prepared Through Solid-State Mechanochemical Method. Polym. Compos. 2017, 40, E177–E185. [Google Scholar] [CrossRef]
- Choudhury, A. Dielectric and piezoelectric properties of polyetherimide/BaTiO3 nanocomposites. Mater. Chem. Phys. 2010, 121, 280–285. [Google Scholar] [CrossRef]
- Hemeda, O.M.; Tawfik, A.; El-Shahawy, M.M.; Darwish, K.A. Enhancement of piezoelectric properties for [poly(vinylidene fluoride)/barium zirconate titanate] nanocomposites. Eur. Phys. J. Plus. 2017, 132, 333. [Google Scholar] [CrossRef]
- Tuff, W.; Manghera, P.; Tilghman, J.; Van Fossen, E.; Chowdhury, S.; Ahmed, S.; Banerjee, S. BaTiO3–Epoxy–ZnO-Based Multifunctional Composites: Variation in Electron Transport Properties due to the Interaction of ZnO Nanoparticles with the Composite Microstructure. J. Electron. Mater. 2019, 48, 4987–4996. [Google Scholar] [CrossRef] [Green Version]
- Kandpal, M.M.; Sharan, C.; Poddar, P.; Prashanthi, K.; Apte, R.; Rao, V.R. Photopatternable nano-composite (SU-8/ZnO) thin films for piezo-electric applications. Appl. Phys. Lett. 2012, 101, 104102. [Google Scholar] [CrossRef]
- Zviagin, A.S.; Chernozem, R.V.; Surmeneva, M.A.; Pyeon, M.; Frank, M.; Ludwig, T.; Tutacz, P.; Ivanov, Y.F.; Mathur, S.; Surmeneva, M.A. Enhanced piezoelectric response of hybrid biodegradable 3D poly(3-hydroxybutyrate) scaffolds coated with hydrothermally deposited ZnO for biomedical applications. Eur. Polym. J. 2019, 117, 272–279. [Google Scholar] [CrossRef]
- Singh, H.H.; Singh, S.; Khare, N. Enhanced β-phase in PVDF polymer nanocomposite and its application for nanogenerator. Polym. Adv. Technol. 2017, 29, 143–150. [Google Scholar] [CrossRef]
- Shen, Z.-Y.; Li, J.-F. Enhancement of piezoelectric constant d33 in BaTiO3 ceramics due to nano-domain structure. J. Ceram. Soc. Jpn. 2010, 118, 940–943. [Google Scholar] [CrossRef] [Green Version]
- Praveen, E.; Murugan, S.; Jayakumar, K. Investigations on the existence of piezoelectric property of a bio-polymer—Chitosan and its application in vibration sensors. RSC Adv. 2017, 7, 35490–35495. [Google Scholar] [CrossRef] [Green Version]
- Hänninen, A.; Sarlin, E.; Lyyra, I.; Salpavaara, T.; Kellomäki, M.; Tuukkanen, S. Nanocellulose and chitosan based films as low cost, green piezoelectric materials. Carbohydr. Polym. 2018, 202, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Van den Ende, D.A.; Bory, B.F.; Groen, W.A.; Van der Zwaag, S. Improving the d33 and g33 properties of 0-3 piezoelectric composites by Dielectrophoresis. J. Appl. Phys. 2010, 107, 024107. [Google Scholar] [CrossRef]






| Polymer Nanocomposite | NPs Dimension (nm) | d33 (pC/N) | Refs |
|---|---|---|---|
| ZnO, bulk | - | 0.4–12.4 | [53,54] |
| photo-epoxy/ZnO films | Less 100 | 15–23 | [78] |
| PVDF/ZnO films | 50–80 | 13.42 | [55] |
| PVDF/ZnO nanoporous films | 35–45 | 18.3 | [56] |
| PVDF β-phase/ZnO | 50–150 | 50 | [54] |
| PVDF-PTTE/ZnO nanorods | - | 70.3 | [57] |
| PHB/ZnO scaffolds | 80–100 | 13.7 | [79] |
| PVDF/ZnO nanorods | - | −1.17 | [80] |
| CS/ZnO films | 40 | 65.9 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokhorov, E.; Luna-Bárcenas, G.; Yáñez Limón, J.M.; Gómez Sánchez, A.; Kovalenko, Y. Chitosan-ZnO Nanocomposites Assessed by Dielectric, Mechanical, and Piezoelectric Properties. Polymers 2020, 12, 1991. https://doi.org/10.3390/polym12091991
Prokhorov E, Luna-Bárcenas G, Yáñez Limón JM, Gómez Sánchez A, Kovalenko Y. Chitosan-ZnO Nanocomposites Assessed by Dielectric, Mechanical, and Piezoelectric Properties. Polymers. 2020; 12(9):1991. https://doi.org/10.3390/polym12091991
Chicago/Turabian StyleProkhorov, Evgen, Gabriel Luna-Bárcenas, José Martín Yáñez Limón, Alejandro Gómez Sánchez, and Yuriy Kovalenko. 2020. "Chitosan-ZnO Nanocomposites Assessed by Dielectric, Mechanical, and Piezoelectric Properties" Polymers 12, no. 9: 1991. https://doi.org/10.3390/polym12091991