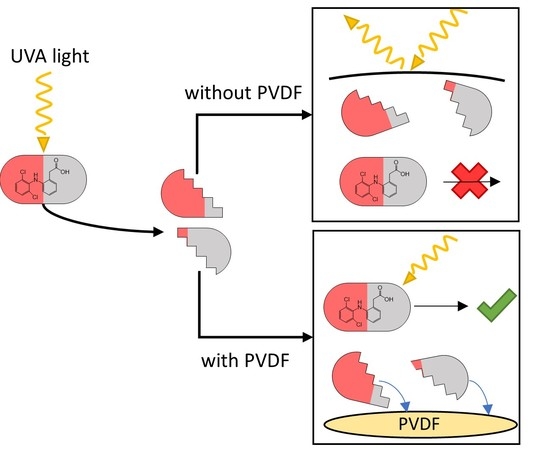
Enhanced Removal and Toxicity Decline of Diclofenac by Combining UVA Treatment and Adsorption of Photoproducts to Polyvinylidene Difluoride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Density Functional Theory Calculations
2.3. Irradiation Experiments
2.4. Characterization of Treated Diclofenac Solution and Extracts
3. Results and Discussion
3.1. Formation of Phototransformation Products and Their Absorption
3.2. Transformation of Diclofenac via UVA Light in the Presence of PVDF
3.2.1. Effect of PVDF
3.2.2. Regeneration of PVDF
3.3. Toxicity Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Der Beek, T.A.; Weber, F.-A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment-Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef]
- Kane, S.P. Diclofenac: Drug Usage Statistics, United States, 2007–2017. Available online: https://clincalc.com/DrugStats/Drugs/Diclofenac (accessed on 11 February 2020).
- Whitacre, D.M. Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2010; Volume 202. [Google Scholar]
- Umwelt Bundesamt. Arzneimittelwirksotffe: Auch die Wirkstoffe von Arzneimitteln können in Gewässern Konzentrationen erreichen, die schädlich auf die aquatische Lebensgemeinschaften wirken. Available online: https://www.umweltbundesamt.de/themen/wasser/fluesse/zustand/arzneimittelwirkstoffe#textpart-1 (accessed on 23 May 2018).
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Pérez, J.A.; Mendoza-Pérez, J.A.; Tintos-Gómez, A.; Ramírez-Ayala, E.; Godínez-Domínguez, E.; Silva-Bátiz, F.D.A. Ecotoxicological Analysis of Emerging Contaminants from Wastewater Discharges in The Coastal Zone of Cihuatlán (Jalisco, Mexico). Water 2019, 11, 1386. [Google Scholar] [CrossRef] [Green Version]
- Ljunggren, B.; Lundberg, K. In vivo phototoxicity of non-steroidal anti-inflammatory drugs evaluated by the mouse tail technique. Photo-Dermatology 1985, 2, 377–382. [Google Scholar]
- The European Commission. Commission Implementing Decision (EU) 2015/495—of 20 March 2015—Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council (notified under document C (2015) 1756); The European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Oaks, J.L.; Gilbert, M.; Virani, M.Z.; Watson, R.T.; Meteyer, C.U.; Rideout, B.A.; Shivaprasad, H.L.; Ahmed, S.; Chaudhry, M.J.I.; Arshad, M.; et al. Diclofenac residues as the cause of vulture population decline in Pakistan. Nat. Cell Biol. 2004, 427, 630–633. [Google Scholar] [CrossRef]
- Green, R.E.; Taggart, M.A.; Das, D.; Pain, D.J.; Kumar, C.S.; Cunningham, A.A.; Cuthbert, R. Collapse of Asian vulture populations: Risk of mortality from residues of the veterinary drug diclofenac in carcasses of treated cattle. J. Appl. Ecol. 2006, 43, 949–956. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999−2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [Green Version]
- Paxéus, N. Removal of selected non-steroidal anti-inflammatory drugs (NSAIDs), gemfibrozil, carbamazepine, b-blockers, trimethoprim and triclosan in conventional wastewater treatment plants in five EU countries and their discharge to the aquatic environment. Water Sci. Technol. 2004, 50, 253–260. [Google Scholar] [CrossRef]
- Kosjek, T.; Heath, E.; Kompare, B. Removal of pharmaceutical residues in a pilot wastewater treatment plant. Anal. Bioanal. Chem. 2007, 387, 1379–1387. [Google Scholar] [CrossRef]
- Guedes-Alonso, R.; Montesdeoca-Esponda, S.; Pacheco-Juárez, J.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. A Survey of the Presence of Pharmaceutical Residues in Wastewaters. Evaluation of Their Removal using Conventional and Natural Treatment Procedures. Molecules 2020, 25, 1639. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Yu, Y.; Tang, C.; Zhang, K.; Cui, J.; Peng, X. Occurrence and behavior of non-steroidal anti-inflammatory drugs and lipid regulators in wastewater and urban river water of the Pearl River Delta, South China. J. Environ. Monit. 2011, 13, 855–863. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Gonçalves, V.M.; Maia, A.S.; Carvalho, M.F.; Castro, P.; Tiritan, M.E. Microbial degradation of pharmaceuticals followed by a simple HPLC-DAD method. J. Environ. Sci. Heal. Part A 2012, 47, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Schweizerische Bundeskanzlei. Verordnung des UVEK zur Überprüfung des Reinigungseffekts von Massnahmen zur Elimination von Organischen Spurenstoffen bei Abwasserreinigungsanlagen, 2016; Schweizerische Bundeskanzlei: Bern, Switzerland, 2016. [Google Scholar]
- Criegee, R. Mechanism of Ozonolysis. Angew. Chem. Int. Ed. 1975, 14, 745–752. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Drinking Water Treatability Database: Granular Activated Carbon. Available online: https://iaspub.epa.gov/tdb/pages/treatment/treatmentOverview.do?treatmentProcessId=2074826383 (accessed on 21 February 2020).
- Verband Schweizer Abwasser- und Gewässerschutzfachleute. Abklärung Verfahreinseignung Ozonung. Empfehlung. 2017. Available online: https://micropoll.ch/Mediathek/abklaerungen-verfahrenseignung-ozonung-empfehlung/ (accessed on 21 February 2020).
- Altmann, J.; Ruhl, A.S.; Zietzschmann, F.; Jekel, M. Direct comparison of ozonation and adsorption onto powdered activated carbon for micropollutant removal in advanced wastewater treatment. Water Res. 2014, 55, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Boehler, M.; Zwickenpflug, B.; Hollender, J.; Ternes, T.; Joss, A.; Siegrist, H. Removal of micropollutants in municipal wastewater treatment plants by powder-activated carbon. Water Sci. Technol. 2012, 66, 2115–2121. [Google Scholar] [CrossRef]
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New perspectives for Advanced Oxidation Processes. J. Environ. Manag. 2017, 195, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Saratale, R.G.; Ghodake, G.S.; Shinde, S.K.; Cho, S.-K.; Saratale, G.D.; Pugazhendhi, A.; Bharagava, R.N. Photocatalytic activity of CuO/Cu(OH)2 nanostructures in the degradation of Reactive Green 19A and textile effluent, phytotoxicity studies and their biogenic properties (antibacterial and anticancer). J. Environ. Manag. 2018, 223, 1086–1097. [Google Scholar] [CrossRef]
- Casillas, J.E.; Campa-Molina, J.; Tzompantzi, F.; Arizaga, G.G.C.; López-Gaona, A.; Ulloa-Godínez, S.; Cano, M.; Barrera, A. Photocatalytic Degradation of Diclofenac Using Al2O3-Nd2O3 Binary Oxides Prepared by the Sol-Gel Method. Materials 2020, 13, 1345. [Google Scholar] [CrossRef] [Green Version]
- Saeid, S.; Kråkström, M.; Tolvanen, P.; Kumar, N.; Eränen, K.; Mikkola, J.-P.; Kronberg, L.; Eklund, P.; Aho, A.; Palonen, H.; et al. Pt Modified Heterogeneous Catalysts Combined with Ozonation for the Removal of Diclofenac from Aqueous Solutions and the Fate of by-Products. Catalysts 2020, 10, 322. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M.O.; Kumar, R.; Ansari, S.P.; Hassan, M.S.A.-W.; Alshahrie, A.; Barakat, M.A.E.-F. Nanocarbon aerogel composites. In Nanocarbon and its Composites; Woodhead Publishing Series in Composites Science and Engineering; Khan, A., Jawaid, M., Inamuddin, Asiri, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 1–26. [Google Scholar]
- Kudlek, E. Decomposition of Contaminants of Emerging Concern in Advanced Oxidation Processes. Water 2018, 10, 955. [Google Scholar] [CrossRef] [Green Version]
- Andreozzi, R.; Raffaele, M.; Nicklas, P. Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere 2003, 50, 1319–1330. [Google Scholar] [CrossRef]
- Fischer, K.; Kühnert, M.; Gläser, R.; Schulze, A. Photocatalytic degradation and toxicity evaluation of diclofenac by nanotubular titanium dioxide–PES membrane in a static and continuous setup. RSC Adv. 2015, 5, 16340–16348. [Google Scholar] [CrossRef] [Green Version]
- Packer, J.L.; Werner, J.J.; Latch, D.E.; McNeill, K.; Arnold, W.A. Photochemical fate of pharmaceuticals in the environment: Naproxen, diclofenac, clofibric acid, and ibuprofen. Aquat. Sci. 2003, 65, 342–351. [Google Scholar] [CrossRef]
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Denney, R.C. A Dictionary of Spectroscopy, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1982; ISBN 9780471874782. [Google Scholar]
- Tarr, M.A. Chemical Degradation Methods for Wastes and Pollutants: Environmental and Industrial Applications (Environmental Science & Pollution Book 26); Tarr, M.A., Ed.; CRC Press: Boca Raton, FL, USA, 2003; ISBN 0824743075. [Google Scholar]
- Young, A.R.; Claveau, J.; Rossi, A.B. Ultraviolet radiation and the skin: Photobiology and sunscreen photoprotection. J. Am. Acad. Dermatol. 2017, 76, S100–S109. [Google Scholar] [CrossRef] [Green Version]
- Encinas, S.; Bosca, F.; Miranda, M.A. Phototoxicity Associated with Diclofenac: A Photophysical, Photochemical, and Photobiological Study on the Drug and Its Photoproducts. Chem. Res. Toxicol. 1998, 11, 946–952. [Google Scholar] [CrossRef]
- Bartels, P.; Vontumplingjr, W. Solar radiation influence on the decomposition process of diclofenac in surface waters. Sci. Total. Environ. 2007, 374, 143–155. [Google Scholar] [CrossRef]
- Eriksson, J.; Svanfelt, J.; Kronberg, L. A Photochemical Study of Diclofenac and Its Major Transformation Products. Photochem. Photobiol. 2010, 86, 528–532. [Google Scholar] [CrossRef]
- Keen, O.S.; Thurman, E.M.; Ferrer, I.; Dotson, A.D.; Linden, K.G. Dimer formation during UV photolysis of diclofenac. Chemosphere 2013, 93, 1948–1956. [Google Scholar] [CrossRef]
- Agüera, A.; Estrada, L.A.P.; Ferrer, I.; Thurman, E.M.; Malato, S.; Fernández-Alba, A.R. Application of time-of-flight mass spectrometry to the analysis of phototransformation products of diclofenac in water under natural sunlight. J. Mass. Spectrom. 2005, 40, 908–915. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.M.; Liu, G.G.; Chen, X.L.; Jiang, K. Photodegradation of diclofenac in aqueous solution by simulated sunlight irradiation: Kinetics, thermodynamics and pathways. Water Sci. Technol. 2017, 75, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Grimm, M.; Meyers, J.; Dietrich, C.; Gläser, R.; Schulze, A. Photoactive microfiltration membranes via directed synthesis of TiO2 nanoparticles on the polymer surface for removal of drugs from water. J. Membr. Sci. 2015, 478, 49–57. [Google Scholar] [CrossRef]
- Paredes, L.; Murgolo, S.; Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Carballa, M.; Mascolo, G. Application of immobilized TiO2 on PVDF dual layer hollow fibre membrane to improve the photocatalytic removal of pharmaceuticals in different water matrices. Appl. Catal. B Environ. 2019, 240, 9–18. [Google Scholar] [CrossRef]
- Altintas, Z.; Chianella, I.; Da Ponte, G.; Paulussen, S.; Gaeta, S.; Tothill, I.E. Development of functionalized nanostructured polymeric membranes for water purification. Chem. Eng. J. 2016, 300, 358–366. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Comments and Replies on Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [Green Version]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Schrodinger. Jaguar, version 10.3; Schrodinger Inc.: New York, NY, USA, 2019. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Tannor, D.J.; Marten, B.; Murphy, R.; Friesner, R.A.; Sitkoff, D.; Nicholls, A.; Honig, B.; Ringnalda, M.; Goddard, W.A. Accurate First Principles Calculation of Molecular Charge Distributions and Solvation Energies from Ab Initio Quantum Mechanics and Continuum Dielectric Theory. J. Am. Chem. Soc. 1994, 116, 11875–11882. [Google Scholar] [CrossRef]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]
- Kovacic, M.; Perisic, D.J.; Biosic, M.; Kusic, H.; Babić, S.; Bozic, A.L. UV photolysis of diclofenac in water; kinetics, degradation pathway and environmental aspects. Environ. Sci. Pollut. Res. 2016, 23, 14908–14917. [Google Scholar] [CrossRef]
- Chianese, S.; Iovino, P.; Leone, V.; Musmarra, D.; Prisciandaro, M. Photodegradation of Diclofenac Sodium Salt in Water Solution: Effect of HA, NO3− and TiO2 on Photolysis Performance. Water Air Soil Pollut. 2017, 228, 1319. [Google Scholar] [CrossRef]
- Persoone, G.; Marsalek, B.; Blinova, I.; Törökne, A.; Zarina, D.; Manusadzianas, L.; Nalecz-Jawecki, G.; Tofan, L.; Stepanova, N.; Tothova, L.; et al. A practical and user-friendly toxicity classification system with microbiotests for natural waters and wastewaters. Environ. Toxicol. 2003, 18, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Cleuvers, M. Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicol. Environ. Saf. 2004, 59, 309–315. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Drozdek, E.; Boruta, T.; Foszpańczyk, M.; Olak-Kucharczyk, M.; Żyłła, R.; Gmurek, M. Impact of Hydrogen Peroxide on the UVC Photolysis of Diclofenac and Toxicity of the Phototransformation Products. Int. J. Photoenergy 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Hanamoto, S.; Kawakami, T.; Nakada, N.; Yamashita, N.; Tanaka, H. Evaluation of the photolysis of pharmaceuticals within a river by 2 year field observations and toxicity changes by sunlight. Environ. Sci. Process. Impacts 2014, 16, 2796–2803. [Google Scholar] [CrossRef] [Green Version]
- Schmitt-Jansen, M.; Bartels, P.; Adler, N.; Altenburger, R. Phytotoxicity assessment of diclofenac and its phototransformation products. Anal. Bioanal. Chem. 2006, 387, 1389–1396. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, K.; Sydow, S.; Griebel, J.; Naumov, S.; Elsner, C.; Thomas, I.; Abdul Latif, A.; Schulze, A. Enhanced Removal and Toxicity Decline of Diclofenac by Combining UVA Treatment and Adsorption of Photoproducts to Polyvinylidene Difluoride. Polymers 2020, 12, 2340. https://doi.org/10.3390/polym12102340
Fischer K, Sydow S, Griebel J, Naumov S, Elsner C, Thomas I, Abdul Latif A, Schulze A. Enhanced Removal and Toxicity Decline of Diclofenac by Combining UVA Treatment and Adsorption of Photoproducts to Polyvinylidene Difluoride. Polymers. 2020; 12(10):2340. https://doi.org/10.3390/polym12102340
Chicago/Turabian StyleFischer, Kristina, Stephan Sydow, Jan Griebel, Sergej Naumov, Christian Elsner, Isabell Thomas, Amira Abdul Latif, and Agnes Schulze. 2020. "Enhanced Removal and Toxicity Decline of Diclofenac by Combining UVA Treatment and Adsorption of Photoproducts to Polyvinylidene Difluoride" Polymers 12, no. 10: 2340. https://doi.org/10.3390/polym12102340