Effect of Ultrasonic and Microwave Dual-Treatment on the Physicochemical Properties of Chestnut Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Samples
2.2.1. Ultrasonic Sample Preparation
2.2.2. Microwave Sample Preparation
2.2.3. Ultrasonic-Microwave and Microwave-Ultrasonic Samples Preparation
2.3. Morphological Structure of Chestnut Starch Granules
2.4. X-ray Diffraction (XRD)
2.5. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
2.6. Thermal Properties
2.7. Pasting Properties
2.8. Swelling Power (SP)
2.9. Freeze-Thaw Stability
2.10. Water Absorption Capacity (WAC) and Oil Absorption Capacity (OAC)
2.11. Statistical Analysis
3. Results and Discussion
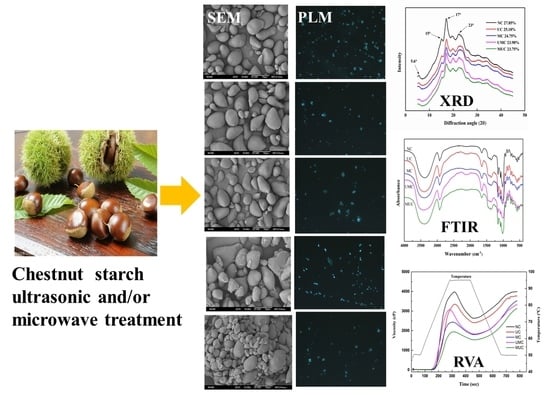
3.1. Morphological Structure
3.2. Long- and Short-Range Molecular Order
3.3. Thermal Properties
3.4. Pasting Properties of Chestnut Starches
3.5. Swelling Power
3.6. Freeze-Thaw Stability
3.7. Water (WAC) and Oil (OAC) Absorption Capacities
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, J.Y.; Kong, L.Y.; Du, B.; Xu, B.J. Morphological and physicochemical characterization of starches isolated from chestnuts cultivated in different regions of China. Int. J. Biol. Macromol. 2019, 130, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, T.; Hu, G.; Guo, K.; Wei, C. Comparison of Physicochemical Properties of Starches from Nine Chinese Chestnut Varieties. Molecules 2018, 23, 3248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.Y.; Lu, X.X.; Chen, Y.Z.; Luo, Z.G.; Xiao, Z.G. Fine structure, crystalline and physicochemical properties of waxy corn starch treated by ultrasound irradiation. Ultrason. Sonochem. 2019, 51, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Xu, M.; Tian, J.; Li, B. Impact of continuous and repeated dry heating treatments on the physicochemical and structural properties of waxy corn starch. Int. J. Biol. Macromol. 2019, 135, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Cruz, B.R.; Abraão, A.S.; Lemos, A.M.; Nunes, F.M. Chemical composition and functional properties of native chestnut starch (Castanea sativa Mill). Carbohydr. Polym. 2013, 94, 594–602. [Google Scholar] [CrossRef]
- Liu, C.; Wang, S.; Chan, X.; Wang, S. Structural and functional properties of starches from Chinese chestnuts. Food Hydrocoll. 2015, 43, 568–576. [Google Scholar] [CrossRef]
- Kaur, B.; Ariffin, F.; Bhat, R.; Karim, A.A. Progress in starch modification in the last decade. Food Hydrocoll. 2012, 26, 398–404. [Google Scholar] [CrossRef]
- Zhu, F.; Li, H. Modification of quinoa flour functionality using ultrasound. Ultrason. Sonochem. 2019, 52, 305–310. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Saleem, S.; Khan, M.U.A.; Nishan, U.; Rasheed, T.; Bilale, M.; Iqbalf, H.M.N. Characteristics of starch isolated from microwave heat treated lotus (Nelumbo nucifera) seed flour. Int. J. Biol. Macromol. 2018, 113, 219–226. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, W.; Pu, H.; Blennow, A.; Liu, X.; Guo, D. Short-time microwave treatment affects the multi-scale structure and digestive properties of high-amylose maize starch. Int. J. Biol. Macromol. 2019, 137, 870–877. [Google Scholar] [CrossRef]
- Colussia, R.; Kringel, D.; Kaur, L.; Zavarezeb, E.R.; Diasb, A.R.G.; Singh, J. Dual modification of potato starch: Effects of heat-moisture and high pressure treatments on starch structure and functionalities. Food Chem. 2020, 318, 126475. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.S.; Mohan, R.J.; Parimalavalli, R. Effect of single and dual-modifications on stability and structural characteristics of foxtail millet starch. Food Chem. 2019, 271, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, X.; Wang, Z.; Bao, Q.; Zhou, B.; Li, T.; Li, S. Preparation and characterization of highly lipophilic modified potato starch by ultrasound and freeze-thaw treatments. Ultrason. Sonochem. 2020, 64, 105054. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.N.; Li, Q.; Bao, W.J.; Wu, Y.W.; Ouyang, J. Relationship between physicochemical characteristics and in vitro digestibility of chestnut (Castanea mollissima) starch. Food Hydrocoll. 2018, 84, 193–199. [Google Scholar] [CrossRef]
- Wang, M.; Sun, M.Q.; Zhang, Y.Y.; Chen, Y.; Wu, Y.W.; Ouyang, J. Effect of microwave irradiation-retrogradation treatment on the digestive and physicochemical properties of starches with different crystallinity. Food Chem. 2019, 298, 125015. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yu, J. In vitro digestion and physicochemical characteristics of corn starch mixed with amino acid modified by heat-moisture treatment. Food Hydrocoll. 2018, 77, 720–725. [Google Scholar] [CrossRef]
- Nara, S.; Komiya, T. Studies on the relationship between water-satured state and crystallinity by the diffraction method for moistened potato starch. Starch-Stärke 1983, 35, 407–410. [Google Scholar] [CrossRef]
- Kaur, H.; Gill, B.S. Effect of high-intensity ultrasound treatment on nutritional, rheological and structural properties of starches obtained from different cereals. Int. J. Biol. Macromol. 2019, 126, 367–375. [Google Scholar] [CrossRef]
- Joshi, M.; Aldred, P.; Mcknight, S.; Panozzo, J.F.; Kasapis, S.; Adhikari, R.; Adhikaria, B. Physicochemical and functional characteristics of lentil starch. Carbohydr. Polym. 2013, 92, 1484–1496. [Google Scholar] [CrossRef]
- Zhao, A.Q.; Yu, L.; Yang, M.; Wang, C.J.; Wang, M.M.; Bai, X. Effects of the combination of freeze-thawing and enzymatic hydrolysis on the microstructure and physicochemical properties of porous corn starch. Food Hydrocoll. 2018, 83, 465–472. [Google Scholar] [CrossRef]
- Deka, D.; Sit, N. Dual modification of taro starch by microwave and other heat moisture treatments. Int. J. Biol. Macromol. 2016, 92, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Sun, S.; Lin, H.; Chen, L.; Qin, S.; Wu, W.; Zheng, B.; Guo, Z. Physicochemical properties and digestion of the lotus seed starch-green tea polyphenol complex under ultrasound-microwave synergistic interaction. Ultrason. Sonochem. 2019, 52, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yan, M.; Yuan, S.; Sun, S.; Huo, Q. Effect of microwave treatment on the physicochemical properties of potato starch granules. Chem. Cent. J. 2013, 7, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambigaipalan, P.; Hoover, R.; Donner, E.; Liu, Q.; Jaiswal, S.; Chibbar, R.; Nantanga, K.K.M.; Seetharaman, K. Structure of faba bean, black bean and pinto bean starches at different levels of granule organization and their physicochemical properties. Food Res. Int. 2011, 44, 2962–2974. [Google Scholar] [CrossRef]
- Ding, Y.; Luo, F.; Lin, Q. Insights into the relations between the molecular structures and digestion properties of retrograded starch after ultrasonic treatment. Food Chem. 2019, 294, 248–259. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, T.; Li, N.; Cheng, Q.; Qiao, D.; Zhang, B.; Zhao, S.; Huang, Q.; Lin, Q. Supramolecular structure and pasting/digestion behaviors of rice starches following concurrent microwave and heat moisture treatment. Int. J. Biol. Macromol. 2019, 135, 437–444. [Google Scholar] [CrossRef]
- Ma, Z.; Yin, X.; Hu, X.; Li, X.; Liu, L.; Boye, J.I. Structural characterization of resistant starch isolated from Laird lentils (Lens culinaris) seeds subjected to different processing treatments. Food Chem. 2018, 263, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Li, Y.; Zheng, J. Dual-frequency ultrasonic effect on the structure and properties of starch with different size. LWT Food Sci. Technol. 2019, 106, 254–262. [Google Scholar] [CrossRef]
- Monroy, Y.; Rivero, S.; García, M.A. Microstructural and techno-functional properties of cassava starch modified by ultrasound. Ultrason. Sonochem. 2018, 42, 795–804. [Google Scholar] [CrossRef]
- Song, H.Y.; Lee, S.Y.; Choi, S.J.; Kim, K.M.; Kim, J.S.; Han, G.J.; Moon, T.W. Digestibility and physicochemical properties of granular sweet potato starch as affected by annealing. Food Sci. Biotechnol. 2014, 23, 23–31. [Google Scholar] [CrossRef]
- Park, D.J.; Han, J.A. Quality controlling of brown rice by ultrasound treatment and its effect on isolated starch. Carbohydr. Polym. 2016, 137, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Tu, Z.; Liu, C.; Liu, W.; Xu, X.; Ai, Y.; Liu, W.; Chen, J.; Wu, J. Effect of microwave irradiation on composition, structure and properties of rice (Oryza sativa L.) with different milling degrees. J. Cereal Sci. 2013, 58, 228–233. [Google Scholar] [CrossRef]
- Flores-Silva, P.C.; Alvarez-Ramirez, J.; Bello-Perez, L.A. Effect of dual modification order with ultrasound and hydrothermal treatments on starch digestibility. Starch-Stärke 2018, 70, 1700284. [Google Scholar] [CrossRef]
- Cooke, D.; Gidley, M.J. Loss of crystalline and molecular order during starch gelatinization: Origin of the enthalpy transition. Carbohydr. Res. 1992, 227, 103–112. [Google Scholar] [CrossRef]
- Shah, U.; Gani, A.; Ashwar, B.A.; Shah, A.; Wani, I.A.; Masoodi, F.A. Effect of infrared and microwave radiations on properties of Indian Horse Chestnut starch. Int. J. Biol. Macromol. 2016, 84, 166–173. [Google Scholar] [CrossRef]
- Bashir, K.; Aggarwal, M. Physicochemical, thermal and functional properties of gamma irradiated chickpea starch. Int. J. Biol. Macromol. 2017, 97, 426–433. [Google Scholar] [CrossRef]
- Pinto, V.Z.; Vanier, N.L.; Deon, V.G.; Moomand, K.; Halal, S.L.M.E.; Zavareze, E.D.R.; Lim, L.K.; Dias, A.R.G. Effect of single and dual physical modifications on pinhão starch. Food Chem. 2015, 187, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.X.; Chen, B.Y.; Zeng, H.L.; Guo, Z.B.; Lu, X.; Zhang, Y.; Zheng, B. Effect of microwave irradiation on the physicochemical and digestive properties of lotus seed starch. J. Agric. Food Chem. 2016, 64, 2442–2449. [Google Scholar] [CrossRef]
- Charoenrein, S.; Preechathammawong, N. Effect of waxy rice flour and cassava starch on freeze-thaw stability of rice starch gels. Carbohydr. Polym. 2012, 90, 1032–1037. [Google Scholar] [CrossRef]
- Gani, A.; Gazanfar, T.; Jan, R.; Wani, S.M.; Masoodi, F.A. Effect of gamma irradiation on the physicochemical and morphological properties of starch extracted from lotus stem harvested from Dal lake of Jammu and Kashmir, India. J. Saudi Soc. Agric. Sci. 2013, 12, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Alimi, B.A.; Workneh, T.S. Structural and physicochemical properties of heat moisture treated and citric acid modified acha and iburu starches. Food Hydrocoll. 2018, 81, 449–455. [Google Scholar] [CrossRef]
- Ashwar, B.A.; Gan, A.; Wani, I.A.; Shah, A.; Masoodi, F.A.; Saxena, D.C. Production of resistant starch from rice by dual autoclaving-retrogradation treatment: Invitro digestibility, thermal and structural characterization. Food Hydrocoll. 2016, 56, 108–117. [Google Scholar] [CrossRef]


| Samples | To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) | IR Ratio of 1047/1022 cm−1 |
|---|---|---|---|---|---|
| NC | 59.8 | 64.2 | 68.0 | 2.91 | 1.1319 |
| UC | 57.5 | 62.2 | 66.6 | 2.61 | 1.1117 |
| MC | 59.8 | 64.0 | 67.8 | 2.30 | 1.2568 |
| UMC | 61.8 | 64.3 | 67.2 | 0.97 | 1.0734 |
| MUC | 59.9 | 63.1 | 66.8 | 1.18 | 1.1212 |
| Samples | 55 °C | 65 °C | 75 °C | 85 °C | 95 °C |
|---|---|---|---|---|---|
| NC | 2.28 ± 0.33 a,b | 7.31 ± 0.12 a | 13.08 ± 0.13 a | 18.09 ± 1.44 a | 31.83 ± 0.14 a |
| UC | 2.32 ± 0.11 b | 7.99 ± 0.04 a | 11.75 ± 0.02 b | 15.76 ± 1.47 b | 26.33 ± 0.42 b |
| MC | 2.74 ± 0.25 a | 8.43 ± 0.57 a | 11.21 ± 0.04 b,c | 14.88 ± 0.53 b | 24.31± 0.13 c |
| UMC | 2.52 ± 0.05 a | 7.46 ± 0.44 a | 9.65 ± 0.34 d | 15.57 ± 0.01 b | 20.54 ± 0.14 d |
| MUC | 2.86 ± 0.05 a | 7.82 ± 0.19 a | 10.36 ± 0.15 c,d | 18.15 ± 0.03 a | 22.81 ± 0.51 c |
| Samples | Syneresis (%) | WAC (g/g) | OAC (g/g) | ||||
|---|---|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | |||
| NC | 0.72 ± 0.04 a | 2.17 ± 0.10 a | 3.81 ± 0.13 a | 8.27 ± 0.02 a | 11.59 ± 0.17 a | 1.05 ± 0.15 d | 1.23 ± 0.04 b |
| UC | 0.42 ± 0.03 b | 0.51 ± 0.06 b | 1.13 ± 0.05 b | 2.48 ± 0.17 b | 3.66 ± 0.10 b | 1.46 ± 0.03 c,d | 1.40 ± 0.04 a,b |
| MC | 0.63 ± 0.06 a | 0.82 ± 0.09 b | 1.02 ± 0.04 b | 1.33 ± 0.04 c | 1.67 ± 0.03 c | 1.76 ± 0.09 b,c | 1.57 ± 0.17 a,b |
| UMC | 0.17 ± 0.01 c | 0.70 ± 0.06 b | 0.98 ± 0.05 b | 1.35 ± 0.10 c | 1.91 ± 0.08 c | 2.14 ± 0.05 a,b | 1.69 ± 0.14 a |
| MUC | 0.68 ± 0.02 a | 0.79 ± 0.02 b | 0.88 ± 0.04 b | 0.97 ± 0.01 c | 1.00 ± 0.03 d | 2.37 ± 0.01 a | 1.37 ± 0.13 a,b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Wu, Y.; Liu, Y.; Ouyang, J. Effect of Ultrasonic and Microwave Dual-Treatment on the Physicochemical Properties of Chestnut Starch. Polymers 2020, 12, 1718. https://doi.org/10.3390/polym12081718
Wang M, Wu Y, Liu Y, Ouyang J. Effect of Ultrasonic and Microwave Dual-Treatment on the Physicochemical Properties of Chestnut Starch. Polymers. 2020; 12(8):1718. https://doi.org/10.3390/polym12081718
Chicago/Turabian StyleWang, Meng, Yanwen Wu, Yongguo Liu, and Jie Ouyang. 2020. "Effect of Ultrasonic and Microwave Dual-Treatment on the Physicochemical Properties of Chestnut Starch" Polymers 12, no. 8: 1718. https://doi.org/10.3390/polym12081718