Anatase Incorporation to Bioactive Scaffolds Based on Salmon Gelatin and Its Effects on Muscle Cell Growth
Abstract
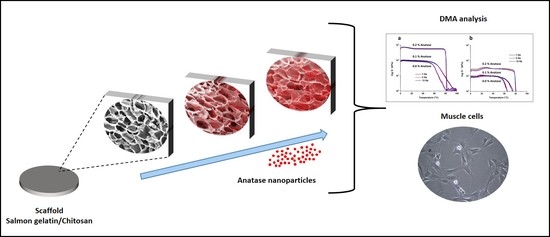
:1. Introduction
2. Materials and Methods
2.1. Scaffold Preparation
2.2. Scaffold Microstructural Characterization
2.3. Differential Scanning Calorimetry (DSC)
2.4. Dynamic Mechanical Analysis (DMA)
2.5. Cell Culture
2.6. Statistical Analysis
3. Results and Discussion
3.1. Microstructure of the Scaffolds
3.2. Thermal Properties of the Scaffolds
3.3. Mechanical Properties of the Scaffolds
3.4. Behavior of Myoblasts Cultured into Scaffolds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, E.J.; Kasper, F.K.; Mikos, A.G. Biomaterials for tissue engineering. Ann. Biomed. Eng. 2014, 42, 323–337. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Ko, H.; Kwon, I.K.; Shin, K. Extracellular matrix revisited: Roles in tissue engineering. Inter. Neurourol. J. 2016, 20, S23–S29. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, C.; Sánchez, E.; Díaz-Calderón, P.; Blaker, J.; Enrione, J.; Quero, F. Synergistic effects of crosslinking and chitosan molecular weight on the microstructure, molecular mobility, thermal and sorption properties of porous chitosan/gelatin/hyaluronic acid scaffolds. J. Appl. Polym. Sci. 2017, 134, 44772. [Google Scholar] [CrossRef]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials. 2019, 12, 1824. [Google Scholar] [CrossRef] [Green Version]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng. B. 2020, 26, 164–180. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.J.; Shin, S.R.; Cha, J.M.; Lee, S.H.; Kim, J.H.; Do, J.T.; Song, H.; Bae, H. Cold water fish gelatin methacryloyl hydrogel for tissue engineering application. PLoS ONE 2016, 11, e0163902. [Google Scholar] [CrossRef] [Green Version]
- Michelini, L.; Probo, L.; Farè, S.; Contessi Negrini, N. Characterization of gelatin hydrogels derived from different animal sources. Mater. Lett. 2020, 272, 127865. [Google Scholar] [CrossRef]
- Ali, E.; Sultana, S.; Hamid, S.B.A.; Hossain, M.; Yehya, W.A.; Kader, A.; Bhargava, S.K. Gelatin controversies in food, pharmaceuticals, and personal care products: Authentication methods, current status, and future challenges. Crit. Rev. Food Sci. Nutr. 2018, 58, 1495–1511. [Google Scholar] [CrossRef]
- Zaupa, A.; Byres, N.; Dal Zovo, C.; Acevedo, C.A.; Angelopoulos, I.; Terraza, C.; Nestle, N.; Abarzua-Illanes, P.N.; Quero, F.; Diaz-Calderon, P.; et al. Cold-adaptation of a methacrylamide gelatin towards the expansion of the biomaterial toolbox for specialized functionalities in tissue engineering. Mater. Sci. Eng C 2019, 102, 373–390. [Google Scholar] [CrossRef]
- Acevedo, C.A.; Sanchez, E.; Orellana, N.; Morales, P.; Olguin, Y.; Brown, D.I.; Enrione, J. Re-epithelialization appraisal of skin wound in a porcine model using a salmon-gelatin based biomaterial as wound dressing. Pharmaceutics 2019, 11, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enrione, J.; Pino, K.; Pepczynska, M.; Brown, D.; Ortiz, R.; Sánchez, E.; Acevedo, C. A novel biomaterial based on salmon-gelatin and its in vivo evaluation as sterile wound-dressing. Mater. Lett. 2018, 212, 159–164. [Google Scholar] [CrossRef]
- MacQueen, L.A.; Alver, C.G.; Chantre, C.O.; Ahn, S.; Cera, L.; Gonzalez, G.M.; O’Connor, B.B.; Drennan, D.J.; Peters, M.M.; Motta, S.E.; et al. Muscle tissue engineering in fibrous gelatin: Implications for meat analogs. NPJ Sci. Food 2019, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhang, X.F.; Gao, G.; Yonezawa, T.; Cui, X. 3d bioprinting and the current applications in tissue engineering. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Griffin, M.A.; Sen, S.; Bonnemann, C.G.; Sweeney, H.L.; Discher, D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004, 166, 877–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, N.; Grover, G.N.; Vincent, L.G.; Evans, S.C.; Choi, Y.S.; Spencer, K.H.; Hui, E.E.; Engler, A.J.; Christman, K.L. A co-culture device with a tunable stiffness to understand combinatorial cell-cell and cell-matrix interactions. Integr. Biol. 2013, 5, 1344–1354. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.H.; Vincent, L.G.; Fuhrmann, A.; Choi, Y.S.; Hribar, K.C.; Taylor-Weiner, H.; Chen, S.; Engler, A.J. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. 2014, 13, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Genchi, G.; Nuhn, H.; Liakos, I.; Marino, A.; Marras, S.; Athanassiou, A.; Mattoli, V.; Desai, T. Titanium dioxide nanotube arrays coated with laminin enhance c2c12 skeletal myoblast adhesion and differentiation. RSC Adv. 2016, 22, 18502–18514. [Google Scholar] [CrossRef]
- Smith, B.S.; Yoriya, S.; Johnson, T.; Popat, K.C. Dermal fibroblast and epidermal keratinocyte functionality on titania nanotube arrays. Acta Biomater. 2011, 7, 2686–2696. [Google Scholar] [CrossRef]
- Liu, H.; Slamovich, E.B.; Webster, T.J. Increased osteoblast functions on nanophase titania dispersed in poly-lactic-co-glycolic acid composites. Nanotechnology 2005, 16, S601–S608. [Google Scholar] [CrossRef]
- Kay, S.; Thapa, A.; Haberstroh, K.M.; Webster, T.J. Nanostructured polymer/nanophase ceramic composites enhance osteoblast and chondrocyte adhesion. Tissue Eng. 2002, 8, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Huerta, F.; Cervantes, B.; Gonzalez, O.; Hernandez-Torres, J.; Garcia-Gonzalez, L.; Vega, R.; Herrera-May, A.L.; Soto, E. Biocompatibility and surface properties of TiO2 thin films deposited by dc magnetron sputtering. Materials 2014, 7, 4105–4117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledo, L.; Racine, L.; Perez, V.; Henriquez, J.P.; Auzely-Velty, R.; Urbano, B.F. Physical nanocomposite hydrogels filled with low concentrations of TiO2 nanoparticles: Swelling, networks parameters and cell retention studies. Mater. Sci. Eng. C Mate Biol. Appl. 2018, 92, 769–778. [Google Scholar]
- Genchi, G.; Cao, Y.; Desai, T. TiO2 nanotube arrays as smart platforms for biomedical applications. Smart Nanopart. Biomed. 2018, 1, 143–157. [Google Scholar]
- Quero, F.; Quintro, A.; Orellana, N.; Opazo, G.; Mautner, A.; Jaque, N.; Valdebenito, F.; Flores, M.; Acevedo, C. Production of biocompatible protein functionalized cellulose membranes by a top-down approach. ACS Biomater. Sci. Eng. 2019, 5, 5968–5978. [Google Scholar] [CrossRef]
- Harzallah, O.; Dupuis, D. Rheological properties of suspensions of TiO2 particles in polymer solutions. 1. Shear viscosity. Rheol. Acta 2003, 42, 10–19. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; O’Brien, F.J. Novel freeze-drying methods to produce a range of collagen-glycosaminoglycan scaffolds with tailored mean pore sizes. Tissue Eng. C 2010, 16, 887–894. [Google Scholar] [CrossRef]
- Aubert, R.; Kenens, B.; Chamtouri, M.; Fujita, Y.; Fortuni, B.; Lu, G.; Hutchison, J.A.; Inose, T.; Uji, I.H. Surface density-of-states engineering of anatase TiO2 by small polyols for enhanced visible-light photocurrent generation. ACS Omega 2017, 2, 6309–6313. [Google Scholar] [CrossRef]
- Yang, L.; Tanabe, K.; Miura, T.; Yoshinari, M.; Takemoto, S.; Shintani, S.; Kasahara, M. Influence of lyophilization factors and gelatin concentration on pore structures of atelocollagen/gelatin sponge biomaterial. Dent. Mater. J. 2017, 36, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Bruzauskaite, I.; Bironaite, D.; Bagdonas, E.; Bernotiene, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes-different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Singh, D.; Singh, D.; Han, S. Surfactant role in modifying architecture of functional polymeric gelatin scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 951–956. [Google Scholar] [CrossRef]
- Dettin, M.; Zamuner, A.; Naso, F.; Monteleone, A.; Spina, M.; Gerosa, G. Natural scaffolds for regenerative medicine: Direct determination of detergents entrapped in decellularized heart valves. Biomed. Res. Int. 2017, 2017, 9274135. [Google Scholar] [CrossRef] [PubMed]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; Ribeiro da Silva, C.E.; Barthes, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of nanoparticles in tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, I.O.; Liu, X.H.; Smith, L.A.; Ma, P.X. Nanostructured polymer scaffolds for tissue engineering and regenerative medicine. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2009, 1, 226–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H.E. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef] [Green Version]
- Pępczyńska, M.; Díaz-Calderón, P.; Quero, F.; Matiacevich, S.; Char, C.; Enrione, J. Interaction and fragility study in salmon gelatin-oligosaccharide composite films at low moisture conditions. Food Hydrocolloids 2019, 97, 105207. [Google Scholar] [CrossRef]
- Díaz-Calderón, P.; Flores, E.; González-Muñoz, A.; Pepczynska, M.; Quero, F.; Enrione, J. Influence of extraction variables on the structure and physical properties of salmon gelatin. Food Hydrocolloids 2017, 71, 118–128. [Google Scholar] [CrossRef]
- Kolathupalayam Shanmugam, B.; Rangaraj, S.; Subramani, K.; Srinivasan, S.; Aicher, W.K.; Venkatachalam, R. Biomimetic TiO2-chitosan/sodium alginate blended nanocomposite scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2020, 110, 110710. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2d and 3d architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [Green Version]
- Gribova, V.; Gauthier-Rouviere, C.; Albiges-Rizo, C.; Auzely-Velty, R.; Picart, C. Effect of rgd functionalization and stiffness modulation of polyelectrolyte multilayer films on muscle cell differentiation. Acta Biomater. 2013, 9, 6468–6480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroese-Deutman, H.C.; van den Dolder, J.; Spauwen, P.H.; Jansen, J.A. Influence of rgd-loaded titanium implants on bone formation in vivo. Tissue Eng. 2005, 11, 1867–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Vlierberghe, S.; Vanderleyden, E.; Boterberg, V.; Dubruel, P. Gelatin functionalization of biomaterial surfaces: Strategies for immobilization and visualization. Polymers 2011, 3, 114–130. [Google Scholar] [CrossRef]
- Gavriilidis, C.; Laredj, L.; Solinhac, R.; Messaddeq, N.; Viaud, J.; Laporte, J.; Sumara, I.; Hnia, K. The mtm1-ubqln2-hsp complex mediates degradation of misfolded intermediate filaments in skeletal muscle. Nat. Cell Biol. 2018, 20, 198–210. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L.J. The effect of pore size on cell adhesion in collagen-gag scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Enrione, J.; Diaz-Calderon, P.; Weinstein-Oppenheimer, C.R.; Sanchez, E.; Fuentes, M.A.; Brown, D.I.; Herrera, H.; Acevedo, C.A. Designing a gelatin/chitosan/hyaluronic acid biopolymer using a thermophysical approach for use in tissue engineering. Bioprocess Biosyst. Eng. 2013, 36, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, C.A.; Diaz-Calderon, P.; Enrione, J.; Caneo, M.J.; Palacios, C.F.; Weinstein-Oppenheimer, C.; Brown, D.I. Improvement of biomaterials used in tissue engineering by an ageing treatment. Bioprocess Biosyst. Eng. 2015, 38, 777–785. [Google Scholar] [CrossRef]
- Enrione, J.; Blaker, J.J.; Brown, D.I.; Weinstein-Oppenheimer, C.R.; Pepczynska, M.; Olguin, Y.; Sanchez, E.; Acevedo, C.A. Edible scaffolds based on non-mammalian biopolymers for myoblast growth. Materials 2017, 10, 1404. [Google Scholar] [CrossRef] [Green Version]
- Meretoja, V.V.; Rossi, S.; Peltola, T.; Pelliniemi, L.J.; Narhi, T.O. Adhesion and proliferation of human fibroblasts on sol-gel coated titania. J. Biomed. Mater. Res. Part A 2010, 95, 269–275. [Google Scholar] [CrossRef]
- Ortega-Lara, W.; Cortés-Hernández, D.; Best, S.; Brooks, R.; Hernández-Ramírez, A. Antibacterial properties, in vitro bioactivity and cell proliferation of titania-wollastonite composites. Ceram. Int. 2010, 36, 513–519. [Google Scholar] [CrossRef]
- Mozumder, M.S.; Zhu, J.; Perinpanayagam, H. Titania-polymeric powder coatings with nano-topography support enhanced human mesenchymal cell responses. J. Biomed. Mater. Res. A 2012, 100, 2695–2709. [Google Scholar] [CrossRef] [PubMed]



| Anatase (%) | Pore Size (µm) | Pore Shape (Circularity) | Tg (°C) | Tm (°C) | ΔHm (J g−1) |
|---|---|---|---|---|---|
| 0.0 | 208.4 ± 22.0 | 0.805 ± 0.069 | 46.9 ± 1.0 | 68.5 ± 3.0 | 8.1 ± 0.9 |
| 0.1 | 223.6 ± 28.0 | 0.743 ± 0.070 | 55.9 ± 0.5 | 72.3 ± 0.5 | 5.5 ± 0.1 |
| 0.2 | 239.5 ± 21.2 | 0.691 ± 0.112 | 55.8 ± 1.6 | 73.2 ± 1.1 | 5.3 ± 0.5 |
| Anatase (%) | Elastic Modulus (G’) at 37 °C (kPa) | Loss Modulus (G’’) at 37 °C (kPa) | ||||
|---|---|---|---|---|---|---|
| 1 Hz | 5 Hz | 10 Hz | 1 Hz | 5 Hz | 10 Hz | |
| 0.0 | 79.5 ± 4.7 | 84.0 ± 3.9 | 85.5 ± 4.0 | 10.3 ± 0.33 | 10.4 ± 0.35 | 9.6 ± 0.41 |
| 0.1 | 90.5 ± 6.2 | 95.5 ± 6.8 | 97.4 ± 4.8 | 9.8 ± 0.58 | 9.7 ± 0.48 | 9.7 ± 0.21 |
| 0.2 | 537.8 ± 3.9 | 572.3 ± 4.4 | ± 4.8 | 31.6 ± 0.42 | 31.2 ± 0.51 | 30.0 ± 0.32 |
| Anatase (%) | Adhesion (%) | µ (d−1) |
|---|---|---|
| 0 | 62.4 ± 4.69 | 0.692 ± 0.021 |
| 0.1 | 57.42 ± 3.86 | 0.734 ± 0.077 |
| 0.2 | 37.26 ± 2.75 | 0.813 ± 0.078 |
| Control (commercial plastic) | 100.00 ± 2.46 | 0.926 ± 0.042 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acevedo, C.A.; Olguín, Y.; Orellana, N.; Sánchez, E.; Pepczynska, M.; Enrione, J. Anatase Incorporation to Bioactive Scaffolds Based on Salmon Gelatin and Its Effects on Muscle Cell Growth. Polymers 2020, 12, 1943. https://doi.org/10.3390/polym12091943
Acevedo CA, Olguín Y, Orellana N, Sánchez E, Pepczynska M, Enrione J. Anatase Incorporation to Bioactive Scaffolds Based on Salmon Gelatin and Its Effects on Muscle Cell Growth. Polymers. 2020; 12(9):1943. https://doi.org/10.3390/polym12091943
Chicago/Turabian StyleAcevedo, Cristian A., Yusser Olguín, Nicole Orellana, Elizabeth Sánchez, Marzena Pepczynska, and Javier Enrione. 2020. "Anatase Incorporation to Bioactive Scaffolds Based on Salmon Gelatin and Its Effects on Muscle Cell Growth" Polymers 12, no. 9: 1943. https://doi.org/10.3390/polym12091943